Question
(a) If the position of a chlorine ion in a membrane is measured to an accuracy of 1.00 μm , what is its minimum uncertainty in velocity, given its mass is
(b) If the ion has this velocity, what is its kinetic energy in eV, and how does this compare with typical molecular binding energies?
Final Answer
- . The kinetic energy of the chloride ion is many orders of magnitude less than typical molecular binding energies.
Solution video
OpenStax College Physics for AP® Courses, Chapter 29, Problem 63 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
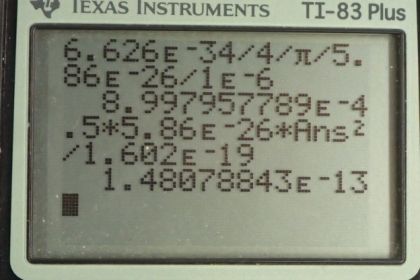
This is College Physics Answers with Shaun Dychko. A chlorine ion has a mass of 5.86 times 10 to the minus 26 kilograms and the uncertainty in measuring its position is 1 micrometer. And the question is, what is the minimum uncertainty therefore in measuring its velocity? Well, the Heisenberg uncertainty principle in equation [29.43] says that the uncertainty in the position multiplied by uncertainty in momentum has to be greater than or equal to Planck's constant divided by 4π. And the uncertainty in momentum can be written as mass times uncertainty in velocity. I mean we are gonna assume that the mass is known perfectly in which case, all of our uncertainty in momentum then can be attributed to uncertainty in velocity. So we'll substitute this in place of Δp. So we have ΔxmΔv is greater than or equal to Planck's constant over 4π and we'll solve for Δv by dividing both sides by Δxm. So the uncertainty in velocity is Planck's constant over 4πmΔx; this is the minimum uncertainty. I mean, to be technically correct, I suppose I should have a greater than or equal to sign here but since the question is asking us to find the minimum uncertainty, well, that's what this is; this is the minimum. So the uncertainty is going to be this or more. OK. So we have Planck's constant divided by 4π times the mass of the chlorine ion multiplied by the uncertainty in its position— 1 times 10 to the minus 6 meters— which gives an uncertainty in velocity of 9 times 10 to the minus 4 meters per second. And now we are told in part (b), suppose that it actually has this velocity, what would its kinetic energy be? So that's 1 half times its mass times its velocity squared. And then we'll convert this into electron volts by multiplying by 1 electron volt for every 1.602 times 10 to the minus 19 joules because we know that this product is gonna give us units of joules since these are mks units here. This gives 1.48 times 10 to the minus 13 electron volts and that is many orders of magnitude less than the binding energy that holds molecules together; the typical molecular binding energy is 10 electron volts and this 13 is orders of magnitude less than that.