Question
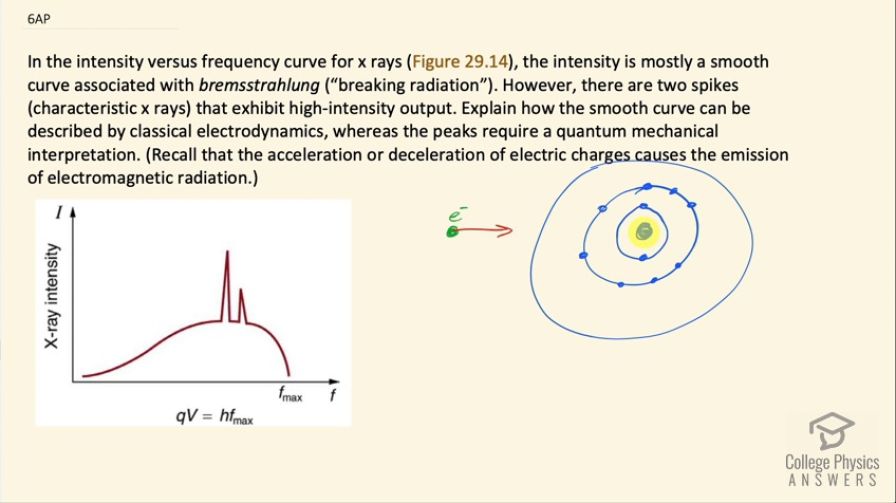
In the intensity versus frequency curve for x rays (Figure 29.14), the intensity is mostly a smooth curve associated with bremsstrahlung (“breaking radiation”). However, there are two spikes (characteristic x rays) that exhibit high-intensity output. Explain how the smooth curve can be described by classical electrodynamics, whereas the peaks require a quantum mechanical interpretation. (Recall that the acceleration or deceleration of electric charges causes the emission of electromagnetic radiation.)
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics for AP® Courses, Chapter 29, Problem 6 (Test Prep for AP® Courses)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. A device that produces x-rays will have electrons that are moving at high velocity and will be hitting some metal in an anode and this picture shows the electron approaching this atom and the speed with which the electron is decelerated determines the frequency of x-ray that it will emit. So it will emit an x-ray due to its deceleration and the greater its deceleration, the greater the frequency of x-ray. So some electrons will approach really close to the nucleus and be decelerated really quickly and others will have a path that takes them farther from the nucleus and they will pass through this mostly empty space through the electron cloud and these different paths cause different rates of deceleration and therefore different x-ray frequencies being emitted and we should expect to see some rather continuous, smooth distribution of frequencies based on a, you know, continuous distribution of paths that one electron can take through this atom. Now everything that I have just said so far has to do with classical electrodynamics; now the part that's quantum mechanical are these peaks here and these peaks are the result of some electrons having just the right path such that they give energy to electrons that are in the orbital of the atom and exciting them and maybe an electron in the s-orbital will get excited up into a d-orbital or something and then it will return back to its ground state and it will emit some photon with an energy equal to the difference between its excited state and its ground state and so this transition from the excited state as a result of being excited by this electron passing through will cause an emission when it returns to the ground state of some photon with a specific frequency, which is this peak that's here and this other peak here corresponds to a different combination of final and initial energy states for electrons in the orbitals around the atoms nucleus. So that notion that only discreet amounts of energy can be absorbed by the atom or emitted that's a quantum mechanical concept and it explains these peaks that are here.