Question
Suppose you are designing a proton decay experiment and you can detect 50 percent of the proton decays in a tank of water. (a) How many kilograms of water would you need to
see one decay per month, assuming a lifetime of ? (b) How many cubic meters of water is this? (c) If the actual lifetime is , how long would you have to wait on an average to see a single proton decay?
Final Answer
- 100 months
Solution video
OpenStax College Physics for AP® Courses, Chapter 33, Problem 49 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
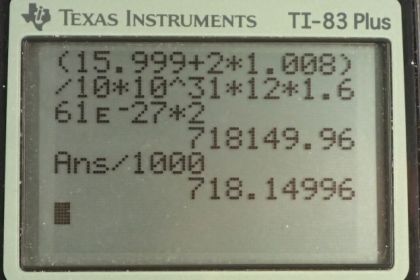
Calculator Screenshots
Video Transcript
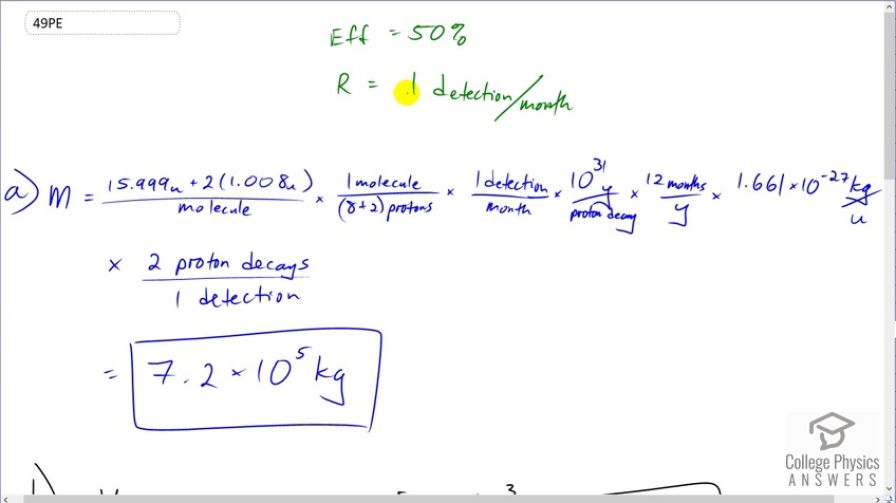
This is College Physics Answers with Shaun Dychko. This question says we have a 50 percent efficiency in detecting proton decays. and we want to detect one proton decay per month and we are asked in part (a), how much mass of water would we need in order to measure one proton decay every month? So I use dimensional analysis to answer questions like this and what that means is I am just looking at units. So we want to have mass and so that means we need to have mass in our numerator. And so I start by finding the mass of a molecule of water so there's the mass of an oxygen atom plus two hydrogen atoms and that's the total mass in atomic mass units per molecule. And in each molecule, there are ten protons so this much mass gives ten protons. And then we multiply that by one detection for every month times 10 to the 31 years per decay and then convert that into months because our detection rate is in units of per months. and the months cancel and the years cancel and we convert the atomic mass units into kilogram so the atomic mass units cancel, the molecules cancel there too and multiply by 1.661 times 10 to the minus 27 kilograms per atomic mass unit and so there we are confirming here that our units are going to be mass in kilograms which is good. And then we multiply by this efficiency of two proton decays that actually happen for every one that is detected and so these detection's cancel proton decays cancel and we are left with this many kilograms in the end and this is just a counting unit that we can ignore. So we have 7.2 times 10 to the 5 kilograms of water are needed in order to measure one proton decay every month. Part (b) asks what volume of water would this be? Well we can take that mass—7.18 times 10 to the 5 kilograms— and multiply it by the density of water which is 1 cubic meter for every 1000 kilograms and we end up with 720 cubic meters. Yeah. Well this is actually not quite density is mass per volume and so volume is mass divided by density I guess that's what I am doing here isn't it but in any case, these units are correct. So when you divide by density which is in units of kilograms per cubic meter, you can multiply it by its reciprocal which is cubic meters per kilogram. OK Then in part (c) it says how long would you have to wait to measure one proton decay if the half-life was 10 to the 33 years instead of 10 to the 31? Well we can see that the detection rate which in this part is one detection per month is inversely proportional to the lifetime of the proton. So if we were to isolate this factor, we would take mass and divide it by every other factor that's here and including this one and we'd see that we have a whole bunch of constants divided by this number— the lifetime of the proton, 10 to the 31. And so we'll summarize all those constants together into this letter c and so the reaction rate in the first case is c divided by the initial half-life which we'll call λ 1. And the second reaction rate that we'll see then is the same numbers; it's the same mass of water and everything else is the same but we are dividing by a new half-life which is 10 to the 33 years so λ 2 is 10 to the 33 which is a hundred times 10 to the 31 and 10 to the 31 is the initial lifetime. So we have 100 λ 1 is something we can substitute in place of λ 2 and so we do that here. And then we can divide R 2 and R 1 and we have c over 100 λ 1 multiplied by the reciprocal of R 2. So actually here it is; it's R 2 is c over 100 λ 1 and then we are multiplying by the reciprocal of R 1 which is λ 1 over c and we end up with 1 over 100. So that is to say that R 2 is R 1 divided by 100 when you multiply both sides by R 1 here. So we expect one detection for every 100 months and so the answer to this question — how many months would you have to wait to notice one detection — that is 100 months.