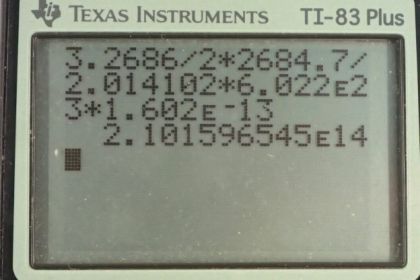
- Yes. For example,
- The energy released by fusion of deuterium in a swimming pool is times greater than that of a gallon of gasoline. Furthermore, there are many orders of magnitude greater mass of water on Earth than gasoline.
Solution video
OpenStax College Physics, Chapter 32, Problem 33 (Problems & Exercises)
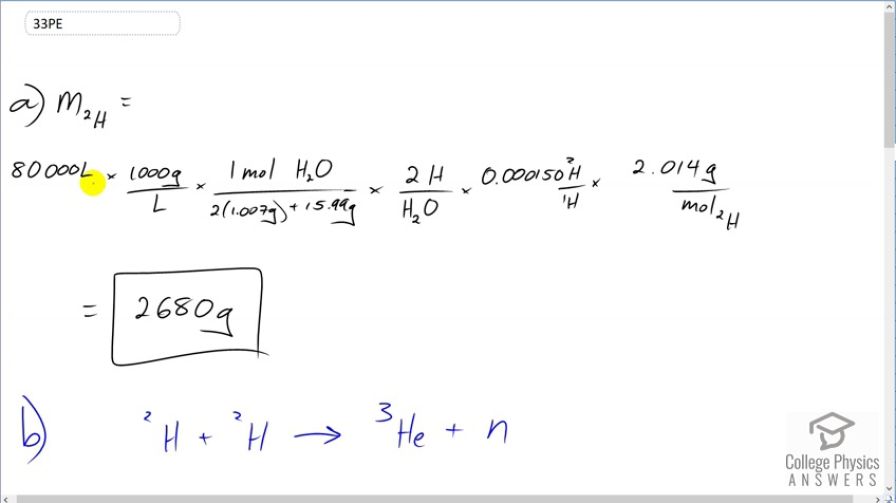
Calculator Screenshots
Comments
I believe there is an error in your calculation. The 2 H/H2O needs to be taken out. With it the mass essentially doubles and then the ratio of hydrogen to the mass of 2 pools is calculated.
Step 1 convert volume to mass. Step 2 calculate the ratio of 2 hydrogen per 1 water molecule using the atomic mass(u) and multiply by the mass of water giving you the mass of all hydrogen in the water. Step 3 multiply the mass of all hydrogen by the percentage of deuterium in the hydrogen mass. Total mass of deuterium is 1.343 kg.
Hi altonbell, thank you for your comment. I almost follow what you're doing, but can't quite see precisely what you method is with words. It might be more helpful to write it out as fractions, ensuring that units cancel such that in the end you're left with mass of deuterium. I see that your answer is half what I've written above so it seems like the point of contention is multiplying by 2 hydrogen per water molecule. I'm not yet convinced there's an error...
All the best,
Shaun