Question
(a) How many nuclei must fission to produce a 20.0-kT yield, assuming 200 MeV per fission? (b) What is the mass of this much ?
Final Answer
Solution video
OpenStax College Physics, Chapter 32, Problem 56 (Problems & Exercises)

vote with a rating of
votes with an average rating of
.
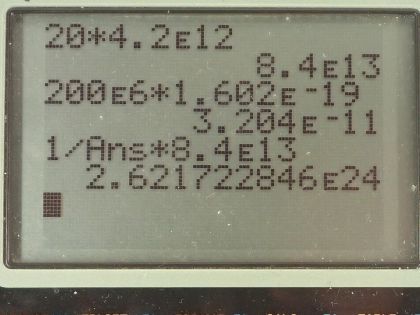
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. How many plutonium-239 nuclei must fission to produce a 20.0 kiloton yield in a bomb? So we will convert the kilotons into joules by multiplying by 4.2 times 10 to the 12 joules per kiloton and that is 8.4 times 10 to the 13 joules. We are told that each fission produces an energy of 200 megaelectron volts and we will convert that into joules by writing 200 times 10 to the 6 electron volts per fission times 1.602 times 10 to the minus 19 joules per electron volt and that is 3.204 times 10 to the minus 11 joules per fission and we are fissioning plutonium-239. So the number of nuclei then will be 1 nucleus per fission— I mean this is maybe a bit obvious but I wrote it down anyway— times 1 fission for every 3.204 times 10 to the minus 11 joules and then multiplied by the number of joules and writing the fractions this way, we can see how the units cancel giving us units of number of nuclei and that's 2.62 times 10 to the 24 nuclei of plutonium-239. How much mass would that number of nuclei have? So we take that number of nuclei, convert it into moles by multiplying by 1 mol for every 6.0221 times 10 to the 23 nuclei— this is Avogadro's number— and then look up in our appendix at the end of the textbook for the molar mass which is 239.052 grams per mol that's 1040 grams or 1.04 kilograms.