Question
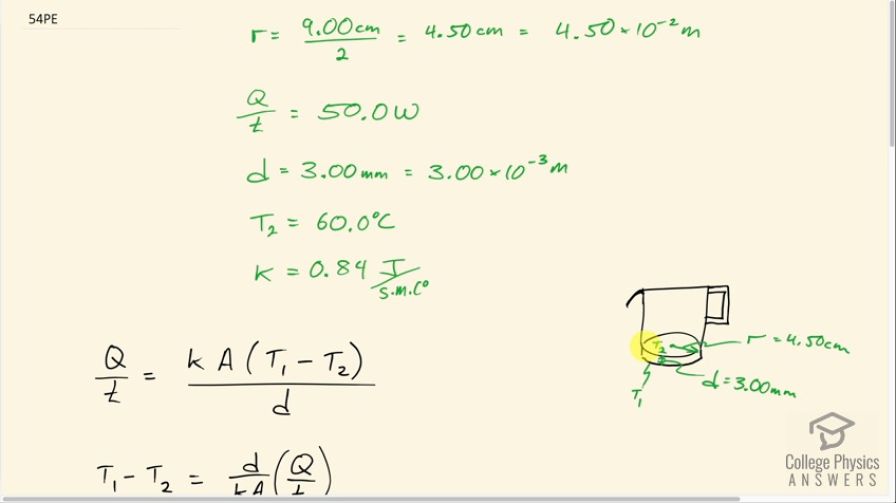
A glass coffee pot has a circular bottom with a 9.00-cm diameter in contact with a heating element that keeps the coffee warm with a continuous heat transfer rate of 50.0 W (a) What is the temperature of the bottom of the pot, if it is 3.00 mm thick and the inside temperature is ? (b) If the temperature of the coffee remains constant and all of the heat transfer is removed by evaporation, how many grams per minute evaporate? Take the heat of vaporization to be 2340 kJ/kg.
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 14, Problem 54 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
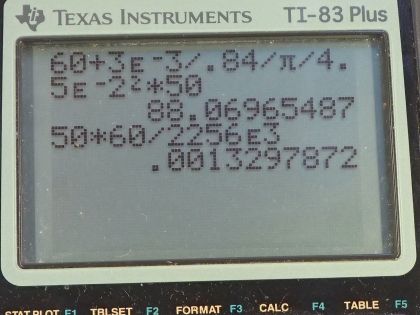
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We have a coffee pot with some hot liquid inside of it and the surface here inside the pot at the bottom of it is at a temperature of 60 degrees Celsius and then 3.00 millimeters further away in contact with the heating element that it's sitting atop of is a surface with temperature T 1. The radius of the bottom of the pot is half the diameter so that's 4.50 centimeters which is 4.50 times 10 to the minus 2 meters and the rate at which heat is entering the pot through this bottom surface is 50 watts or 50 joules per second. We look up the thermal conductivity of glass which is 0.84 joules per second per meter per Celsius degree and our job is to figure out what is this temperature T 1, the temperature of the hot plate element that the coffee pot is sitting on. So the rate of thermal conduction across this bottom of the pot is the thermal conductivity of glass times the area multiplied by the difference in temperatures on both sides divided by the thickness and we are gonna solve for T 1 and we'll get there by multiplying both sides by d over kA and then we have T 1 minus T 2 equals d over kA times Q over t and then we'll add T 2 to both sides. So we get T 1—the temperature of the element—is T 2 plus the thickness of the glass on the bottom divided by the thermal conductivity of the glass multiplied by the area times the rate of heat conduction across the surface. Now the area is that of a circle so it's π times r squared so we substitute that in place of A and now we plug in numbers. So we have 60.0 degrees Celsius plus 3.00 times 10 to the minus 3 meters— that's 3.00 millimeters written in meters— divided by the thermal conductivity of glass times π times the r squared times 50.0 watts and that is 88.1 degrees Celsius is the temperature of the hot plate surface. Now in part (b) we are asked what mass of water is going to evaporate every minute given that the temperature of the water is not changing. So that means an amount of heat is being carried away by evaporation which is equal to the amount of heat that's being absorbed in that time. So the amount of heat being absorbed is the power, which is 50.0 watts, multiplied by time and that's gonna equal the mass of water that evaporates times the latent heat of vaporization. So we'll divide both sides by the latent heat and we get mass is 50.0 watts times 1 minute expressed in seconds in order to match with the mks units here— watts is joules per second; we need the seconds to cancel— and we divide by the latent heat of vaporization. This is at 100.0 degrees Celsius which is a bit... a bit too low of a number because the water is at, you know, 60.0 degrees Celsius but you know we have only data at 37.0 and we have data at 100.0 and so I just chose the one that's at 100.0. If we wanted to be more sophisticated, we could have done a linear extrapolation between this value and the 2430 times 10 to the 3 joules per kilogram at 37.0 degrees Celsius that would have been a more sophisticated approach. Figure out what the slope is of the straight line between this and this with respect to temperature and then figure out at, you know, 60 degrees Celsius, what would the latent heat of vaporization be but anyway... we just took a shortcut and used this number from the data table. This works out to 1.33 grams of water evaporating every minute.