Question
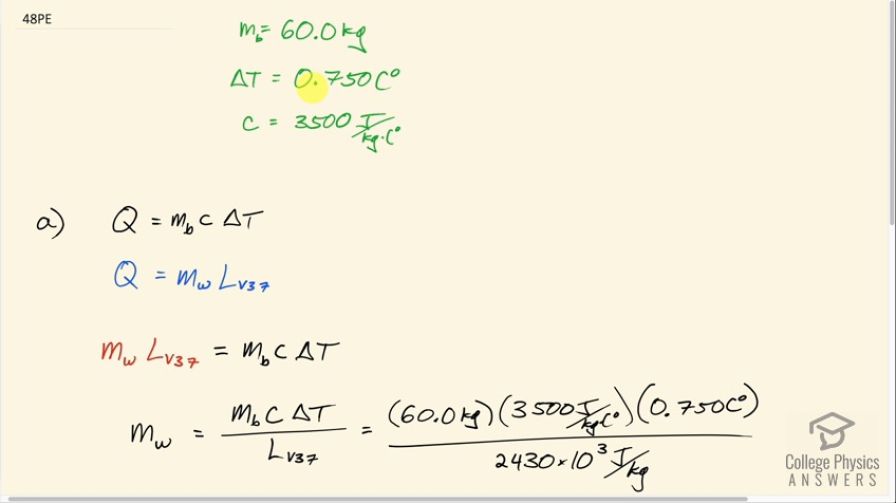
(a) How many kilograms of water must evaporate from a 60.0-kg woman to lower her body temperature by ?
(b) Is this a reasonable amount of water to evaporate in the form of perspiration, assuming the relative humidity of the surrounding air is low?
Final Answer
- Yes, this is a reasonable amount of sweat. 64 mL of sweat is much less than a bottle of water that she might drink to stay hydrated.
Solution video
OpenStax College Physics for AP® Courses, Chapter 14, Problem 48 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. In this question we are going to figure out what mass of water needs to evaporate in order to cool a 60.0 kilogram woman down by 0.750 Celsius degrees. We look up the specific heat of the human body and it's about 3500 joules per kilogram per Celsius degree. Now the amount of heat that the water will need to take away is equal to the mass of the body times the specific heat of the body times its change in temperature and the amount of heat that the water will take away is also equal to the mass of water that evaporates— which is what we want to find— multiplied by the latent heat of vaporization at 37.0 degrees for water. So we equate these two things and substituting this in place of Q and I do that here and then solve for the mass of water by dividing both sides by the latent heat of vaporization. And so the mass of water that has to evaporate is 60.0 kilograms times 3500 joules per kilogram per Celsius degree times 0.750 Celsius degrees divided by 2430 times 10 to the 3 joules per kilogram and that's 64.8 grams and the question (b) asks is this a reasonable amount of water? Well let's turn it into a volume because we can imagine water volumes easier since we buy water bottles and so on labeled with volumes in liters or milliliters... at least in Canada, they are labeled with liters and milliliters. So we have this mass of 64.81 grams times 1 milliliter for every gram and we end up with 64.8 milliliters. And this is a reasonable amount of sweat; it's much less than a bottle of water that she might drink to stay hydrated and provide her body with more water with which to sweat with.
