Question
A man consumes 3000 kcal of food in one day, converting most of it to maintain body temperature. If he loses half this energy by evaporating water (through breathing and sweating), how many kilograms of water evaporate?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 14, Problem 34 (Problems & Exercises)
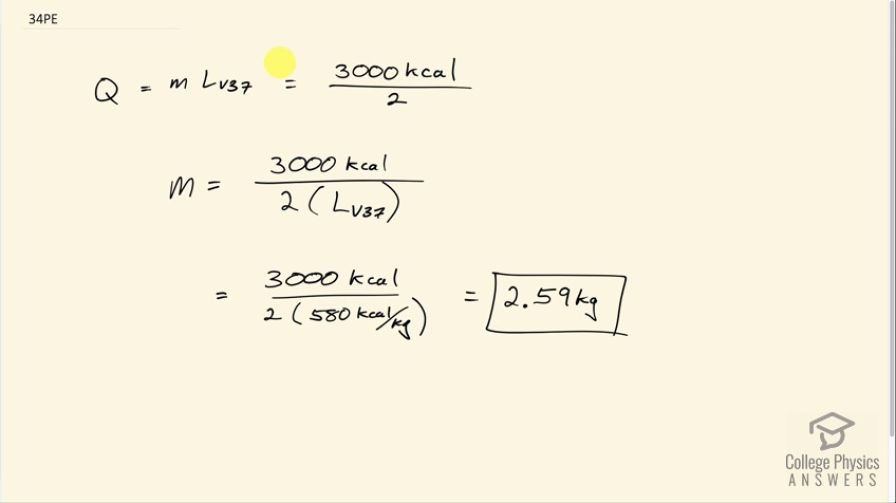
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. This person consumes 3000 kilocalories of food energy and half of that we are told is lost due to the evaporation of water. So the amount of heat absorbed by that water is its mass times the latent heat of vaporization at 37.0 degrees Celsius and we can solve this for m by dividing both sides by latent heat of vaporization and we get that the mass then is 3000 kcal divided by 2 times 580 kilocalories per kilogram and this is 2.59 kilograms of water.
