Question
The yellow light from a sodium vapor lamp seems to be of pure wavelength, but it produces two first-order maxima at and when projected on a 10,000 line per centimeter diffraction grating. What are the two wavelengths to an accuracy of 0.1 nm?
Final Answer
Solution video
OpenStax College Physics, Chapter 27, Problem 29 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
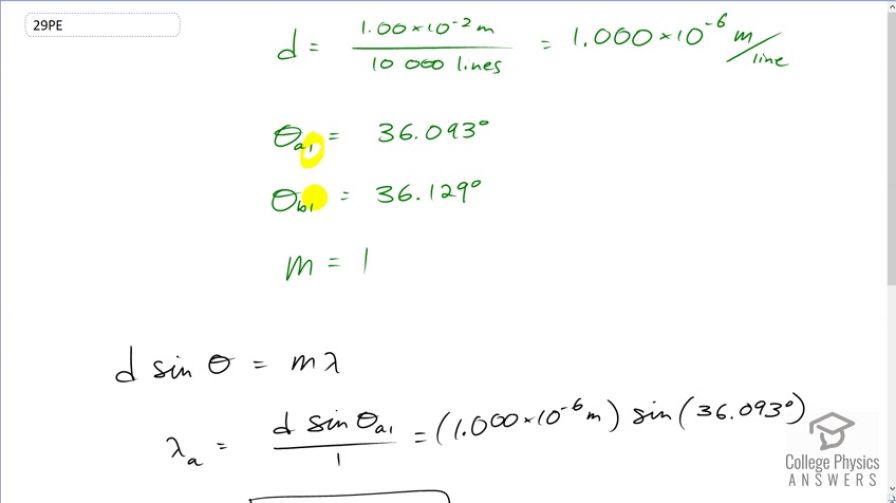
This is College Physics Answers with Shaun Dychko. Light from a sodium vapor lamp has two first order Maxima. This number one denotes the order and the letter a or the letter b denotes the wavelength. So this is the first order angle to the maximum for wavelength a which is 36.093 degrees. And this is the angle to the first order maximum of wavelength b 36.129 degrees. Because the maximum is the first order m is one in our formula for Maxima from a diffraction grating and we also need to figure out what d is. We're told is the diffraction grating has 10,000 lines per centimeter when we can take the reciprocal of that to figure out the number of meters between each line. So that's one times ten to the minus two meters. That's one centimeter expressed in meters. Divided by 10,000 lines is one time ten to the minus six meters between each line. So now we use this formula here to figure out what is each of the wavelengths and we can solve it for lambda by dividing both sides by the order m. So the wavelength is going to be separation between the slits in the diffraction grating times sine of the angle to the maximum divided by the order. So for wavelength a, it's gonna be d times sine theta a one over one. So this is the first order maximum of wavelength a. And that's one time ten to the minus six meters time sine of 36.093 degrees which is 589.1 nanometers and then for wavelength b, It's one times ten to the minus seven meters. Oops, that's a ten to the minus six meters. Times sine of a different angle 36.129 degrees. And that is 589.6 nanometers.
