Question
A 50.0 g ball of copper has a net charge of . What fraction of the copper's electrons has been removed? (Each copper atom has 29 protons, and copper has an atomic mass of 63.5.)
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 18, Problem 7 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
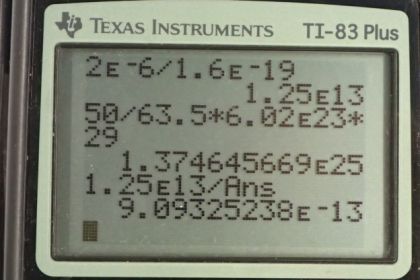
This is College Physics Answers with Shaun Dychko. To find the fraction of electrons removed from this copper ball, we’ll figure out how many electrons were removed and then we’ll figure out how many electrons the ball has when it’s neutral, and then we’ll divide the two. So the electrons removed correspond to the number of electrons in two microcoulombs which is two times ten to the minus six coulombs, and we multiply that by one electron for every 1.6 times ten to the minus 19 coulombs, we’re kind of ignoring negative signs here by the way, and we end up with 1.25 times ten to the 13 electrons. Now the total electrons that it has when it’s neutral is 50 grams, that’s the mass of the ball we’re told, times one mol for every 63.5 grams, using the atomic mass 63.5 there, and then times by Avogadro's number which is 6.02 times ten to the 23 atoms per mol, and then times by 29 electrons for every atom since each atom has 29 protons, that means there are 29 electrons to balance each of those protons in a neutral atom. And we end up with 1.3746 times ten to the 25 electrons. So there’s 1.25 times ten to the 13 electrons that were removed here divided by the total that it has when it’s neutral, represents a fraction of 9.09 times ten to the minus 13.