Question
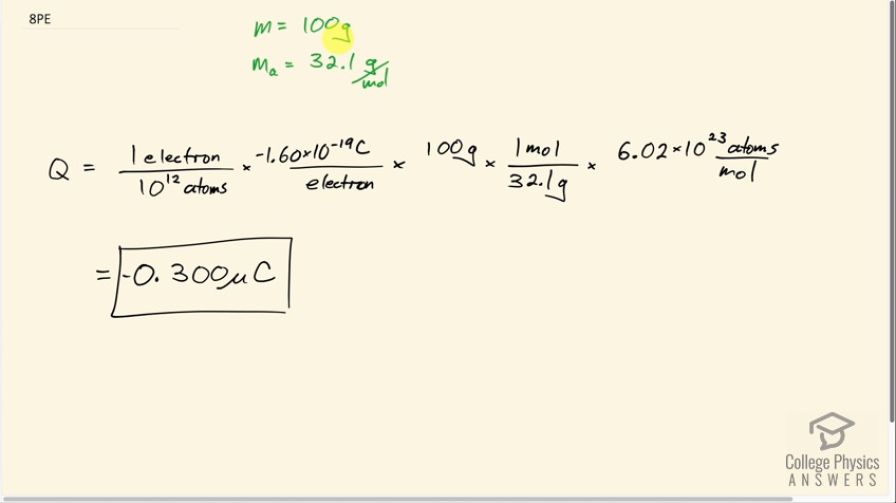
What net charge would you place on a 100 g piece of sulfur if you put an extra electron on 1 in of its atoms? (Sulfur has an atomic mass of 32.1.)
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 18, Problem 8 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. If one electron is placed on every 10 to the 12 atoms in a 100 gram sample of sulphur, what would the net charge be on the sample? The atomic mass of sulphur is 32.1 grams per mole so we are gonna multiply the 1 electron for every 10 to the 12 atoms by the number of coulombs per electron so that's multiplying by negative 1.60 times 10 to the minus 19 coulombs per electron, the electrons cancel and then we want to know how many atoms are there in 100 grams of sulphur? So 100 grams will get multiplied by 1 mole for every 32.1 grams and now we have units of moles here and we want to turn moles into atoms so we multiply by 6.02 times 10 to the 23 atoms per mole and then we have atoms here— they cancel with these atoms— and the only unit we are left with is coulombs. So our strategy as usual here for this... it is kind of an unit conversion... sort of... strategy where we have the units that we want in the numerator or well... I mean units will be wherever they are meant to be but in this case we wanted coulombs and so we needed to have a coulomb in our numerator which we had which we found with this factor here— coulombs per electron—and then we choose other things such that we cancel away the units that we don't want and so we don't want grams, we want number of atoms so we had to multiply the grams by 1 mole for every 32.1 grams and so on and then by Avogadro's number of atoms per mole so units that we don't want got canceled and units that we want are left behind. Negative 0.300 microcoulombs is the net charge on 100 grams of sulphur.
