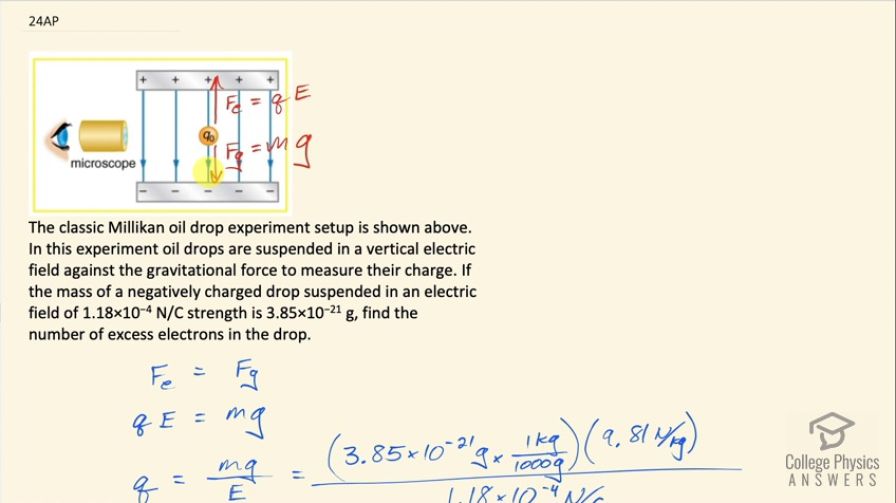
Question
The classic Millikan oil drop experiment setup is shown below. In this experiment oil drops are suspended in a vertical electric field against the gravitational force to measure their charge. If the mass of a negatively charged drop suspended in an electric field of strength is , find the number of excess electrons in the drop.
Final Answer
2 electrons
Solution video
OpenStax College Physics for AP® Courses, Chapter 18, Problem 24 (Test Prep for AP® Courses)
vote with a rating of
votes with an average rating of
.
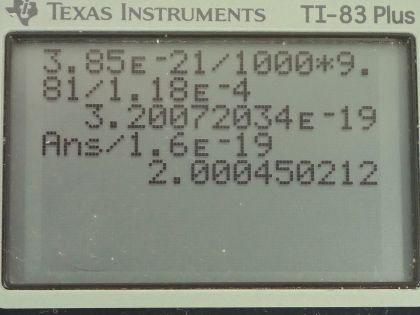
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. The Millikan oil-drop experiment shows this little drop of oil with some charge on it suspended in this electric field and there is an electrostatic force upwards of magnitude charge multiplied by electric field strength that perfectly balances the force of gravity downwards, which is mass times gravitational field strength. So we can say these two things are equal and our job is to find the number of excess electrons in the drop. So the electrostatic force is the charge times electric field strength equals mass times g and we'll solve for q by dividing both sides by E and then this will give us an answer in coulombs for the charge and then we'll convert the coulombs into number of electrons. So we are told that the mass of the drop is 3.85 times 10 to the minus 21 grams which we have to convert into kilograms by multiplying by 1 kilogram for every 1000 grams and then we multiply by 9.81 newtons per kilogram divided by 1.18 times 10 to the minus 4 newtons per coulomb is the field strength and this is 3.20072 times 10 to the minus 19 coulombs. The number of electrons will be that charge in coulombs multiplied by one electron per elementary charge—1.60 times 10 to the minus 19 coulombs— and that is 2 electrons.