Question
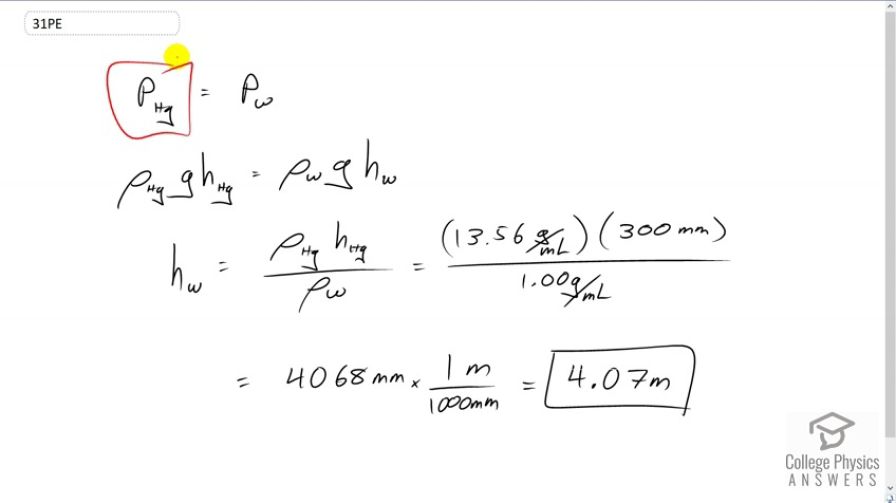
How tall must a water-filled manometer be to measure blood pressures as high as 300 mm Hg?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 11, Problem 31 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We’re asked to find out what is the height of a water column that would be needed to measure a given pressure in millimeters of mercury. So the pressure is the same and the height of the column of mercury gets multiplied by the density of mercury times g to get this pressure. Then that same gauge pressure for water will be the density of water times g times the height of the water column. We’re going to solve for hw by dividing both sides by rho w g, and the gs cancel everywhere and we’re left with height of the water column is density of mercury times the height of the mercury column divided by the density of water, so that’s 13.56 grams per milliliter times 300 millimeters of mercury, we’re told that’s the height of the mercury column, divided by one gram per milliliter, this makes 4068 millimeters which we will convert into meters by dividing by 1000, and we get 4.07 meters of water, will be the height of the water column needed to measure the same pressure that is measured by 300 millimeters of mercury.
