Question
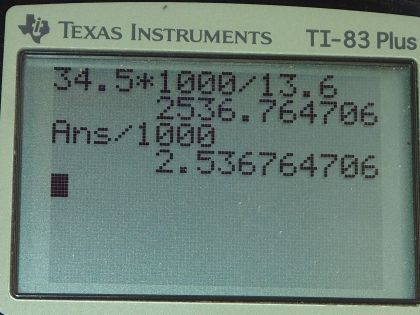
Mercury is commonly supplied in flasks containing 34.5 kg (about 76 lb). What is the volume in liters of this much mercury?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 11, Problem 2 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. Given 34.5 kilograms of mercury, we want to know what the volume of that mass of mercury will be. Now we know density is mass divided by volume and we can find the density of mercury by looking it up in this handy data table [11.1] and the density of mercury is 13.6 grams per milliliter or 13.6 times 10 to the 3 kilograms per cubic meter depending on your preference for units; I am gonna go with grams per milliliter which is why you see 13.6 grams per milliliter written here. So we have rearranged the density formula to solve for V by multiplying both sides by V over ρ and the ρ's cancel and the V's cancel on this side and we are left with V equals mass divided by density. So 34.5 kilograms but since I have written the density units in grams per milliliter, I need to convert the units in the numerator to grams so we'll multiply by 1000 grams per kilogram so now these grams will cancel and that's important. So we are left with milliliters in our answer that's 2536.8 milliliters which we convert into liters by multiplying by 1 liter for every 1000 milliliters and that is 2.54 liters will be the volume of 34.5 kilograms of mercury.