Question
Particles called muons exist in cosmic rays and can be created in particle accelerators. Muons are very similar to electrons, having the same charge and spin, but they have a mass 207 times greater. When muons are captured by an atom, they orbit just like an electron but with a smaller radius, since the mass in is .
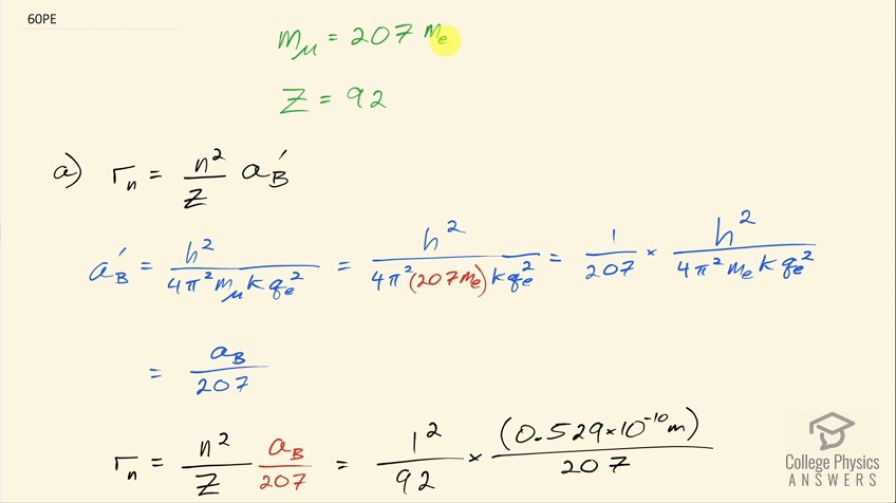
(a) Calculate the radius of the orbit for a muon in a uranium ion ().
(b) Compare this with the 7.5-fm radius of a uranium nucleus. Note that since the muon orbits inside the electron, it falls into a hydrogen-like orbit. Since your answer is less than the radius of the nucleus, you can see that the photons emitted as the muon falls into its lowest orbit can give information about the nucleus.
Final Answer
- times the nuclear radius.
Solution video
OpenStax College Physics, Chapter 30, Problem 60 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A muon has a mass that is 207 times the mass of an electron but is otherwise identical to an electron with the same charge and the same spin and we are asked to figure out what is the radius of the orbit of an electron in the ground state of an uranium ion and we are told that the number of protons in the nucleus of an uranium ion is 92. So here's our formula for the radius in the nth energy level for an electron around a hydrogen-like ion and so you know uranium... we can either assume that it has only one electron on it— it's an ion of charge 91 plus— or we can say, well, the outer electrons don't matter in the same way that outer electrons on a metallic shell or charges on the shell of something that's metallic results in a zero net electric field inside that shell and so all the electrons that are in this atom of uranium don't matter when it comes to the center most orbital since the electric field will cancel from all the different surrounding electrons. Okay! So in other words, we can use this formula to calculate the radius of this electron's orbit. So it's going to be the shell number, which we're told is 1... squared, divided by the number of protons times the Bohr radius but the Bohr radius gets this little prime on here that I added to indicate that it's different from the electron's Bohr radius. The radius for the muon is going to be the same formula as we'd calculate for the Bohr radius for an electron except we have m μ here for the muon mass and so we can replace that with 207 times the electron mass and then bring that factor 1 over 207 out and it's being multiplied by all of this and all of this is the regular Bohr radius a B with no prime divided by 207. So the radius then of the orbital is going to be n squared times the electron Bohr radius divided by 207 times the number of protons. So that's 1 squared over 92 times 0.529 times 10 to the minus 10 meters divided by 207 and that's 2.78 femtometers. In part (b) we are asked to compare this radius for the electron to the radius of the nucleus of uranium and it's strange but true that the muon will have a radius less than that of the nucleus itself, it's going to be 0.370 times the radius of the nucleus.