Question
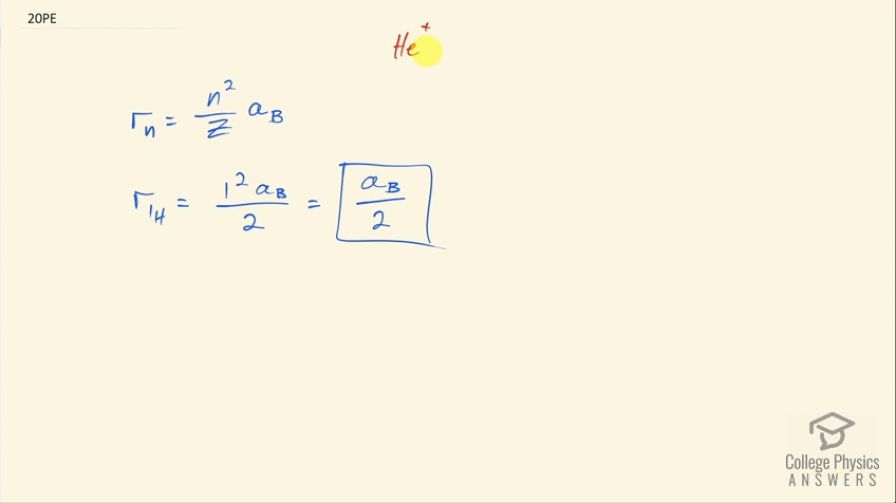
A singly ionized helium ion has only one electron and is denoted . What is the ion’s radius in the ground state compared to the Bohr radius of hydrogen atom?
Final Answer
Solution video
OpenStax College Physics, Chapter 30, Problem 20 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. A helium ion that has a charge of +1 has only one electron surrounding a nucleus with two protons so because it has only one electron, it is considered hydrogen like and so this formula therefore applies for finding the radius of the ion for the nth energy level of the electron. So we are meant to compare the radius of this helium ion with the radius of hydrogen in the ground state and hydrogen has a radius in the ground state with a special name called Bohr radius— that's after Niels Bohr— and it has a special symbol for it, which is a B. So this formula will tell us what the radius of the helium ion is in terms of the Bohr radius. So we plug in the energy level of the electron, which in this case is 1 because it's the ground state so it's the smallest possible number for n, which is 1 and then we divide that by the number of protons in the nucleus, which is 2. So the r 1 for helium is 1 squared times Bohr radius over 2 so that's half the Bohr radius. So the radius of helium ion in the ground state is half the Bohr radius.