Question
Which of the following spectroscopic notations are allowed (that is, which violate none of the rules regarding values of quantum numbers)?
Final Answer
(b) and (d) violate constraints on possible quantum numbers.
Solution video
OpenStax College Physics, Chapter 30, Problem 49 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Video Transcript
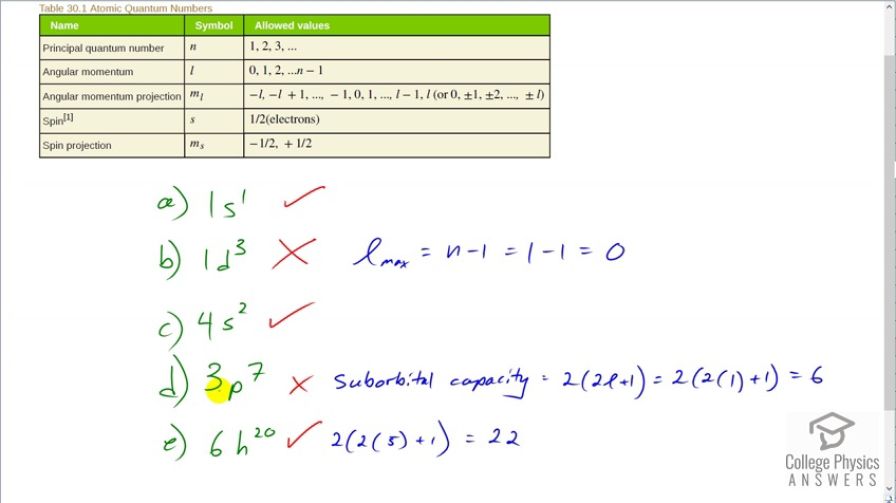
This is College Physics Answers with Shaun Dychko. We have here some of the rules about atomic quantum numbers and here are some spectroscopic notations for electron states and we have to see which ones are impossible. So this means that the principal quantum number is 1 so the spectroscopic notation has the first big number in front is the letter n, principal quantum number and then after that is a letter representing angular momentum quantum number so that's l and then the superscript here is the number of electrons that are in that state. So or in that subshell would be another way to say that so number of electrons in this l-orbital. So this shell is 1 and the orbital is the s-orbital which corresponds to l equals 0 and there's 1 electron in that state and sure it can be 1 electron in any state so definitely this is a possible spectroscopic notation. Part (b) it says that the principal quantum number is 1 and that there is a subshell d, or an orbital d, with three electrons. Now three electrons do fit in the d-orbital but the d-orbital cannot exist with a principal quantum number of 1 because it's constrained by this constraint here where the maximum value of l is 1 less than n. Now with n being 1, that makes the maximum value of l, 0 and so it's at the most l max because n minus 1 which is 1 minus 1 in this case which is 0. And so you can't have a d-orbital which corresponds to an l equals to 2 so this is not a possible state and that's why we have an 'X' right here. OK. Then we are looking at part (c) and we have an s orbital at principal quantum number 4 and it contains 2 electrons and that's fine. So I mean s corresponds to l equal to 0 which is certainly possible because n is well l equals 0 is always possible and the s-orbital can contain 2 electrons; 1 being spin up and other being spin down because with a angular momentum quantum number of 0, the angular momentum projection is going to have to be 0 and in this state of, you know, n l, m l and m s—these are the four quantum numbers— you can have four, zero, zero and this can be either positive a half spin up or it can be negative a half spin down which corresponds to two different electrons and so 2 is OK for that subscript; had this been a 3, we would have said no or more. OK. Part (d) says that the principal quantum number is 3 and the orbital is p which corresponds to l equal to 2 and you cannot have 7 electrons in a p-orbital. Here's a formula for suborbital I should say; here's a formula telling you how many electrons will fit in a suborbital and it is 2 times 2 times the angular momentum quantum number plus 1 so oh I'm sorry, this is l equal to 1 by the way for p yeah so in the spectroscopic notation, it's l and then the symbol 0 is s, 1 is p, 2 is d and 3 is f. OK. So this is 6 is the maximum number of electrons you can have in a p-orbital. So not possible because there's a 7 there. And then in (e), h corresponds to l equal to 5 and with this formula telling you the suborbital capacity, we have 2 times 5 is 10 plus 1 is 11 times 2 makes 22 and so there could be 22 electrons in this suborbital and it's saying 20 there so that's OK and then we have to check to make sure this number 5 is acceptable given principal quantum number 6 and it is acceptable because l at its most can be 1 less than n and n being 6 makes l being 5 is possible.