Question
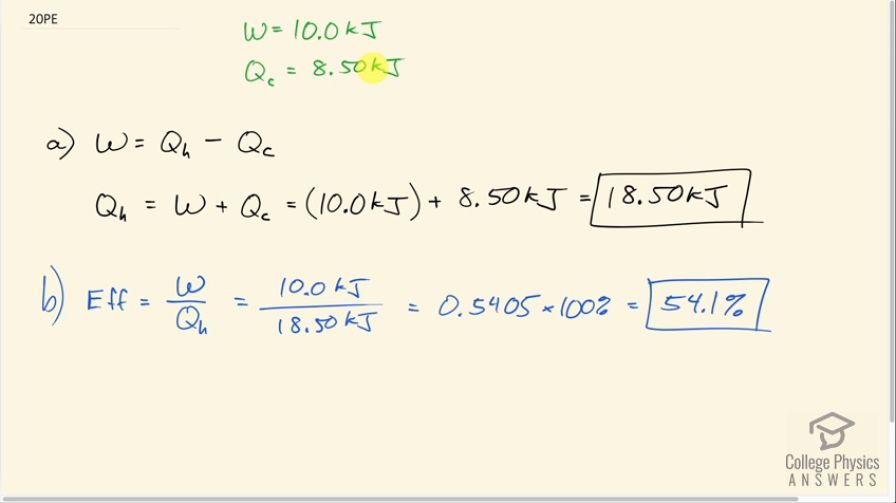
A certain heat engine does 10.0 kJ of work and 8.50 kJ of heat transfer occurs to the environment in a cyclical process. (a) What was the heat transfer into this engine? (b) What was the engine’s efficiency?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 15, Problem 20 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A cyclical heat engine does 10.0 kilojoules of work and it expels 8.50 kilojoules of heat to the environment. Now because it's a cyclical heat engine that means the work done is the heat absorbed from the high temperature reservoir minus the heat expelled to the cold temperature reservoir. So we can solve for Q h which is the total heat absorbed by adding Q c to both sides and so Q h is w plus Q c. So that's 10.0 kilojoules plus 8.50 kilojoules which is 18.50 kilojoules... maybe it should just be 18.5 because this is significant only to the tenths place. Okay! Efficiency is the work done divided by the total heat absorbed so that's 10.0 kilojoules divided by 18.50 kilojoules which is 54.1 percent.
