Question
A hot rock ejected from a volcano's lava fountain cools from to , and its entropy decreases by 950 J/K. How much heat transfer occurs from the rock?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 15, Problem 49 (Problems & Exercises)
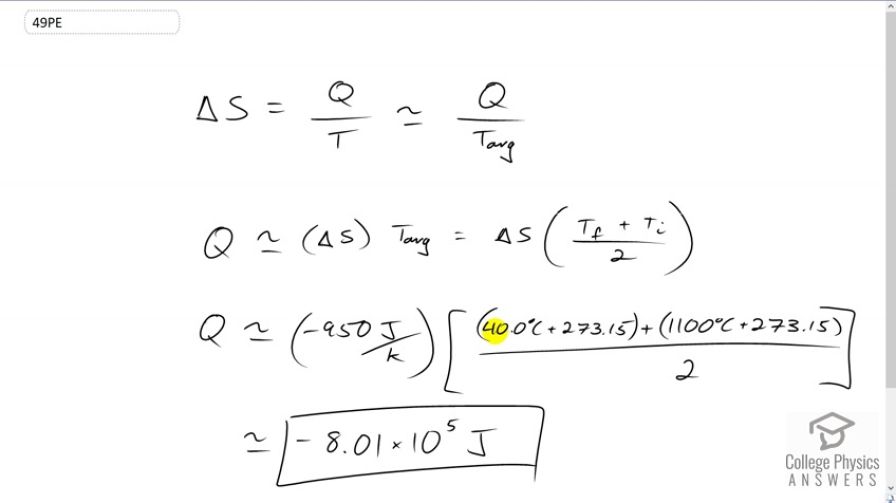
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A hot rock ejected from a volcano has a temperature change from 1100 degrees Celsius to 40 degrees Celsius. And, we're told what the change in entropy is negative 950 Joules per Kelvin. And so the question is: "How much heat energy was released by the rock to the environment?" So, the change in entropy is the amount of heat transferred into the rock divided by its absolute temperature, and that's approximately equal to the heat transferred into it divided by its average temperature. So we'll solve for Q by multiplying both sides by T average. And so Q is change in entropy multiplied by average temperature. So that is, the final temperature plus the initial temperature divided by two, and multiplied by Delta S. Now the heat transferred into the rock is negative because it's actually releasing heat to the environment. And so, we'll say, negative 950 Joules per Kelvin is the change in entropy, times 40 degrees Celsius converted into Kelvin by adding 273.15, plus the initial temperature of 1100 degrees Celsius converted into Kelvin, all divided by two, and this makes negative 8.01 times 10 to the five Joules. And this negative here means there is a negative amount of heat transferred into the rock, which is the same as saying a positive amount of heat transferred to the environment, so to speak.
