Question
(a) How much food energy will a man metabolize in the process of doing 35.0 kJ of work with an efficiency of 5.00%? (b) How much heat transfer occurs to the environment to keep his temperature constant? Explicitly show how you follow the steps in the Problem-Solving Strategy for thermodynamics found in Problem-Solving Strategies for Thermodynamics.
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 15, Problem 6 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
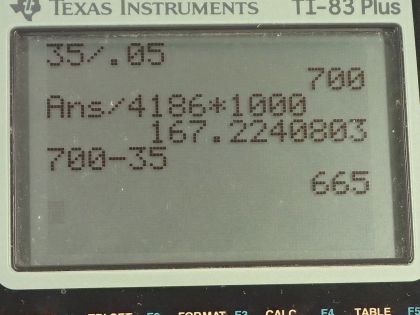
This is College Physics Answers with Shaun Dychko. This man does 35.0 kilojoules of work with an efficiency of 5.00 percent and question (a) asks how much food energy will the man consume in order to do this work? So the efficiency is the useful work done divided by the amount of food energy consumed and we can solve for Q in by multiplying both sides by Q in divided by efficiency and then we have the total energy used is the work done divided by efficiency. So that's 35.0 kilojoules divided by 5.00 percent expressed as a decimal as 0.0500 and that's 700 kilojoules. But it asks for the amount of food energy and so that suggests that the units should be in calories with capital 'C.' So we multiply by 1 food calorie for every 4186 joules and then also convert between the kilojoules to joules here and this also works out to 167 Calories. Now of that... some of those calories are going to be expended as heat and that's what question (b) is asking assuming constant temperature in which case the internal energy is going to be unchanged since it's proportional to temperature; we have Q minus w equals 0, in other words. Q is the heat in minus the heat out and it's this heat lost to the environment that we are interested in. So we replace Q with Q in minus Q out and then we'll add Q out to both sides here and we get Q out is Q in minus work. So that's 700 kilojoules of heat in minus 35.0 joules of work which is 665 kilojoules of heat lost to the environment.