Question
Suppose you want to operate an ideal refrigerator with a cold temperature of , and you would like it to have a coefficient of performance of 7.00. What is the hot reservoir temperature for such a refrigerator?
Final Answer
Solution video
OpenStax College Physics, Chapter 15, Problem 43 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
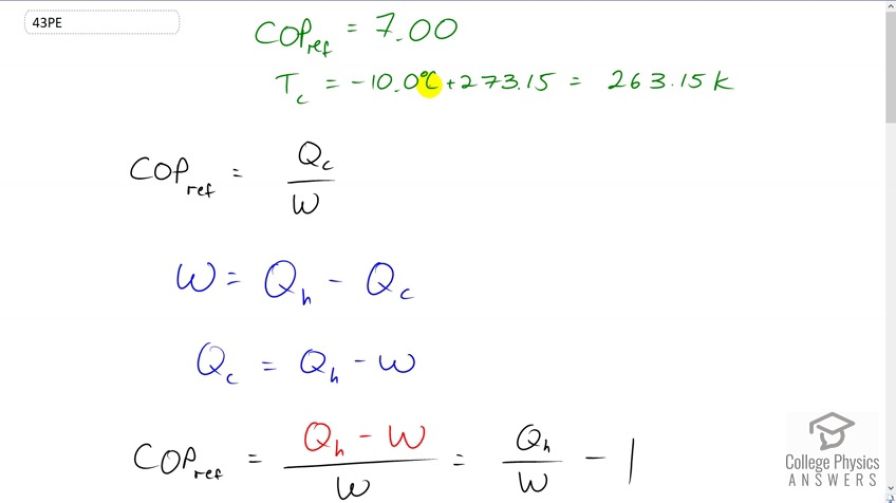
This is College Physics Answers with Shaun Dychko. An ideal refrigerator has a coefficient of performance of 7. And the cold reservoir temperature is minus 10 degrees Celsius, which we convert into Kelvin by adding 273.15. And we're asked to figure out what should the hot temperature be of the high-temperature reservoir. So, coefficient of performance for refrigerator is the amount of heat taken out of the cold temperature reservoir, divided by the amount of work needed to operate that heat pump. Now, work is the difference between the energy that is delivered to the high-temperature reservoir, minus the energy removed from the cold temperature reservoir, and we'll solve this for Qc because we want to express coefficient of performance in terms of Qh, since we have a formula for efficiency in terms of work and Qh. And then we'll use the Carnot efficiency because we're told that this is an ideal refrigerator. And then this Carnot efficiency formula has a Th in it, which we're going to solve for. So, we rearrange this to solve for Qc, by adding Qc to both sides and subtracting W, and we get Qc equals Qh minus W. And we replace Qc in our coefficient of performance formula with Qh minus W. And then divide both terms in the numerator by W and you get Qh over W minus one. That's the coefficient of performance for a refrigerator in terms of Qh. Now, efficiency is work divided by Qh. And so we see that this is the reciprocal of efficiency. So we can replace it with efficiency to the power of negative one. And this isn't just any old efficiency, this is meant to be the Carnot efficiency, and there's a formula for that which is one minus the cold temperature reservoir, divided by the temperature of the hot reservoir. And, we can write this as a single fraction by multiplying this one by Th over Th. So we have Th minus Tc over Th. And so, we plug that in for efficiency and so, coefficient of performance then is Th minus Tc over Th, all to the power of negative one, minus one. And then, this exponent of negative one means flip the fraction, and so we'll write that instead as Th over Th minus Tc. Now there's a bunch of algebra to solve for Th. So, first of all, the common denominator between these two terms, so this one will get multiplied by Th minus Tc over Th minus Tc. And then we have Th minus this bracket Th minus Tc all over Th minus Tc. And then distribute the minus sign into the bracket, makes a negative Th and then a positive Tc, and then the negative Th plus Th makes zero there leaving us only with positive Tc on the numerator. So we have Tc over Th minus Tc. And then, multiply both sides by Th minus Tc, and multiply the coefficient of performance by the same thing. And we have coefficient of performance times Th minus Tc equals Tc. And then, distribute the coefficient of performance into the brackets and you have coefficient of performance times Th, minus coefficient of performance times Tc, equals Tc. And then, add this term to both sides. And then we have, high-temperature, times coefficient of performance equals cold temperature, plus cold temperature, times coefficient of performance. And then divide both sides by this coefficient of performance here, and we finally get a formula. So, the temperature of the hot reservoir is the cold reservoir temperature, times one plus the coefficient of performance, all divided by the coefficient of performance. So that's 263.15, is the absolute temperature in Kelvin, times one plus seven, divided by seven, and this works out to 300.74 Kelvin, which is 27.6 degrees Celsius.
