Question
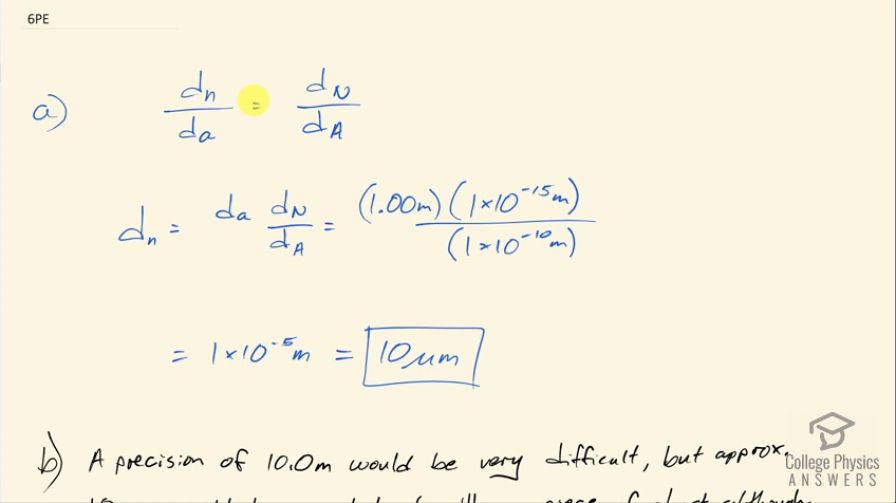
(a) An aspiring physicist wants to build a scale model of a hydrogen atom for her science fair project. If the atom is 1.00 m in diameter, how big should she try to make the nucleus? (b) How easy will this be to do?
Final Answer
- A precision of would be very difficult, but approximately could be accomplished with a piece of small dust, although it would be hard to see.
Solution video
OpenStax College Physics for AP® Courses, Chapter 30, Problem 6 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A physicist wants to make a scale model of the atom with the atom having a size of 1.00 meter so the whole atom is 1.00 meter across and if that's the case, what would the size of the nucleus need to be in order to be drawn to scale? So we have the ratio of the nuclear diameter of the model divided by the diameter of the whole atom has to equal the ratio of the actual real nucleus divided by the diameter of a real atom and we'll solve for the diameter of nucleus then by multiplying both sides by the scale diameter of the atom and so we have 1.00 meter times 10 to the minus 15 meters— nuclear diameter— divided by 10 to the minus 10 meters for a typical atom across and this works out to 10 micrometers and 10 micrometers is possible to create an object with that size like a piece of dust but it would be hard to see and if you want a precision to the tenths place that would be very difficult but you know to the tenths place in micrometers might be possible and there we go!
