Question
Show that (Rydberg's constant), as discussed in the text.
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics for AP® Courses, Chapter 30, Problem 14 (Problems & Exercises)
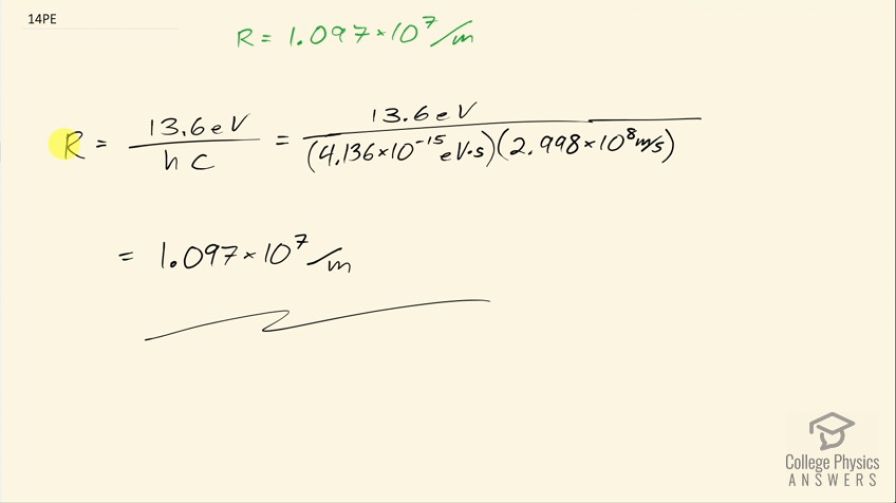
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We are going to verify that the Rydberg's constant is 1.097 times 10 to the 7 per meter. So formula for the Rydberg's constant is 13.6 electron volts divided by Planck's constant times speed of light. So that's 13.6 electron volts divided by Planck's constant written with units of electron volt seconds because that's convenient since these electron volts will cancel so that's 4.136 times 10 to the minus 15 electron volt seconds and the seconds here will cancel with the 'per seconds' in meters per second in the speed of light here so we are multiplying by 2.998 times 10 to the 8 meters per second here and this works out to 1.097 times 10 to the 7 per meter as we expected.
