Question
Suppose an MRI scanner uses 100-MHz radio waves. (a) Calculate the photon energy. (b) How does this compare to typical molecular binding energies?
Final Answer
- The photon energy is about times smaller than typical molecular binding energies.
Solution video
OpenStax College Physics for AP® Courses, Chapter 30, Problem 63 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
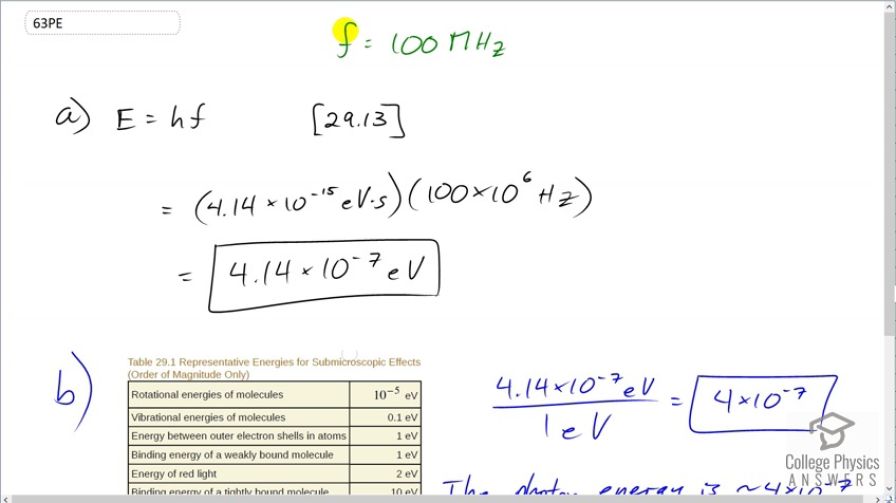
This is College Physics Answers with Shaun Dychko. The energy of a photon is given by equation [29.13] which is Planck's constant times the frequency and we are told the frequency is 100 megahertz. So we go 100 times 10 to the 6 hertz multiplied by Planck's constant— 4.14 times 10 to the minus 15 electron volt seconds— giving an energy of 4.14 times 10 to the minus 7 electron volts. And then we compare that with representative energies for sub-microscopic effects and we see that the binding energy of a weakly bound molecule is about 1 electron volt. And so we take this photon energy, divide it by that and we get 4 times 10 to the minus 7. So the photon energy is about 4 times 10 to the minus 7 times smaller than typical molecular binding energies.
