Question
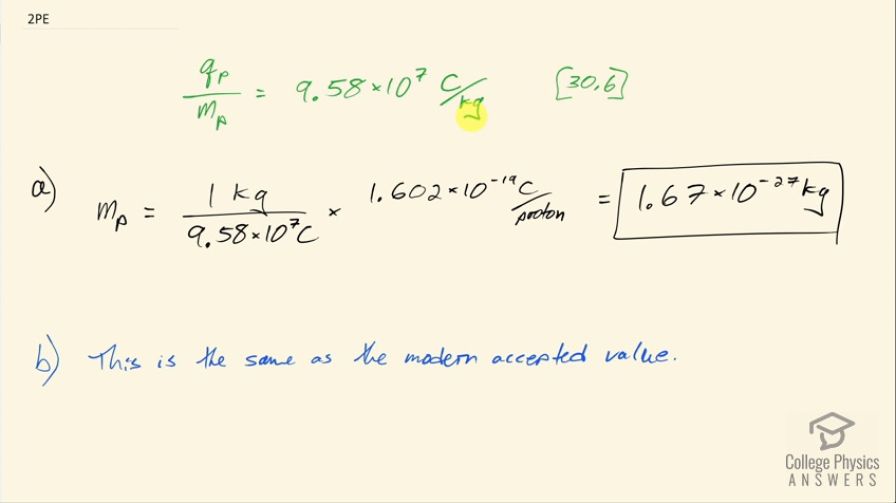
(a) Calculate the mass of a proton using the charge-to- mass ratio given for it in this chapter and its known charge. (b) How does your result compare with the proton mass given in this chapter?
Final Answer
- This is the same as the modern accepted value for the mass of a proton.
Solution video
OpenStax College Physics for AP® Courses, Chapter 30, Problem 2 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. Given the charge to mass ratio for a proton is 9.58 times 10 to the 7 coulombs per kilogram, we can figure out the mass of the proton knowing its charge; it has an elementary charge of 1.602 times 10 to the minus 19 coulombs for every proton and we multiply that by the reciprocal of this ratio which is 1 kilogram for every 9.58 times 10 to the 7 coulombs and we see that the coulombs cancel leaving us with kilograms per proton and that is 1.67 times 10 to the minus 27 kilograms and this is the same as the modern accepted value for the mass of a proton.
