Question
If an atom has an electron in the state with , what are the possible values of ?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 30, Problem 35 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Video Transcript
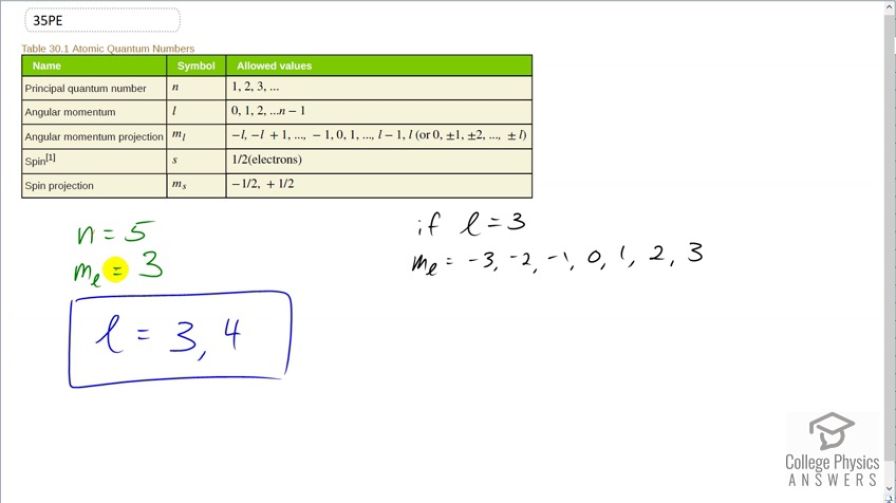
This is College Physics Answers with Shaun Dychko. An atom has a principal quantum number, n, of 5 and the angular momentum projection quantum number of 3 and the question is what are the possible values for the angular momentum quantum number? So if we consider just this constraint— n equals 5— that means that l could be anything between 0, 1, 2, 3 or 4; the maximum number is the principal quantum number minus 1 so the maximum possible number in this sequence is 5 minus 1 which is 4. So these are all the possible angular momentum quantum numbers based on the principal quantum number 5. But we have to add to this the other constraint that the angular momentum projection quantum number is 3. And so with m l being 3, well there's constraints on what m l can be and if l was 3, m l can be starting at negative whatever l is so negative 3 and then increasing by 1's so negative 2, negative 1, 0 and 1, 2 up to a maximum of l which is 3 so hypothetically, if l was 3, that would allow an m l of 3. But if l was 2, m l can be only negative 2, negative 1, 0, 1 or 2 and so with l being 2, m l cannot be 3. So l can't be 2 and it also can't be 1 and it can't be 0 so we can exclude each of these; that leaves us only with 3 and 4 are the possibilities. So l is either 3 or it's 4 and we can't tell which based on the constraints we have but we have narrowed it down to one of those two numbers.