Question
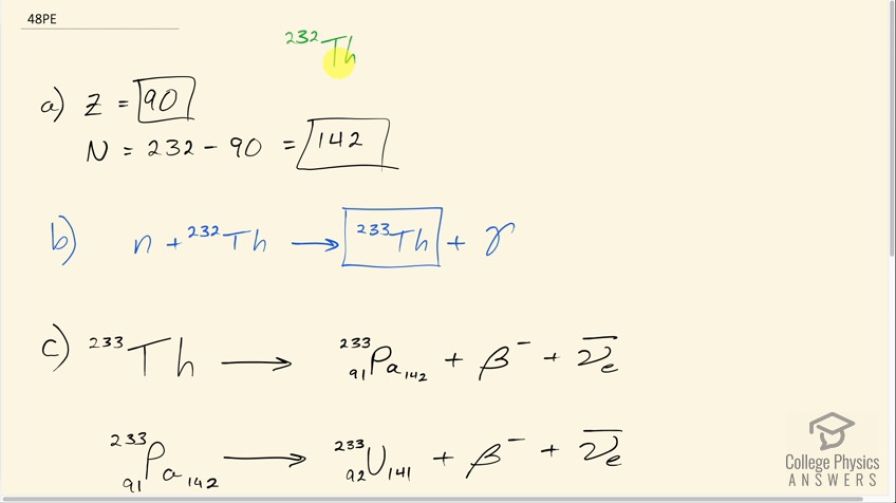
The naturally occurring radioactive isotope does not make good fission fuel, because it has an even number of neutrons; however, it can be bred into a suitable fuel (much as is bred into ).
- What are and for
- Write the reaction equation for neutron capture by and identify the nuclide produced in .
- The product nucleus decays, as does its daughter. Write the decay equations for each, and identify the final nucleus.
- Confirm that the final nucleus has an odd number of neutrons, making it a better fission fuel.
- Look up the half-life of the final nucleus to see if it lives long enough to be a useful fuel.
Final Answer
- ,
- Please see the solution video
- Yes, the final nucleus has an odd number of neutrons: 141
Solution video
OpenStax College Physics for AP® Courses, Chapter 32, Problem 48 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. Thorium-232 does not make good fission fuel because it has an even number of neutrons and question (a) asks us how many neutrons does it have? Well first we need to know the atomic number which is the number of protons and we look that up in the appendix at the end of the chapter and thorium-232 has 90 protons. So that's what Z is and the number of neutrons then will be the total number of nucleons, which is 232 minus 90 protons which is 142 neutrons; this is an even number which makes this nuclide a bit more stable. Part (b) says write the reaction for neutron capture by thorium-232. So when a neutron enters the nucleus, it's still going to stay as thorium and it's going to have 233 nucleons now because it has 143 neutrons and we are told that there's also a gamma ray released as part of this reaction. And part (c) says this thorium-232 undergoes beta decay and we should break the reaction for that. So if a negative charge is being produced then there must be an additional positive charge to offset this appearance of a new negative charge so that the total charge is the same as we started with— we started with a total charge of 90 protons in the thorium nuclide— and so with an additional negative charge, we have an additional proton for a total of 91 protons and that makes the element protactinium. And there's also an electron anti-neutrino produced to offset the plus 1 electron family number here and the electron anti-neutrino has a negative 1 electron family number for a total of 0 on the right to match the 0 electron family number that we have on the left side of the arrow. The protactinium-233 also undergoes beta decay and thereby produces an extra proton in the nuclide turning it into uranium and still the same number of nucleons—233— so this is uranium-233 that's produced now along with a beta particle and an electron anti-neutrino. Part (d) asks to confirm that the final nucleus has an odd number of neutrons so the number of neutrons then is the total number of nucleons—233— minus the 92 protons, which is 141 neutrons and that is an odd number. The half-life of this nuclide I had to look up in Wikipedia and it's 1.6 times 10 to the 5 years which means it can be used as reactor fuel because you know, this material won't just disappear right away, it has quite a long half-life.