Question
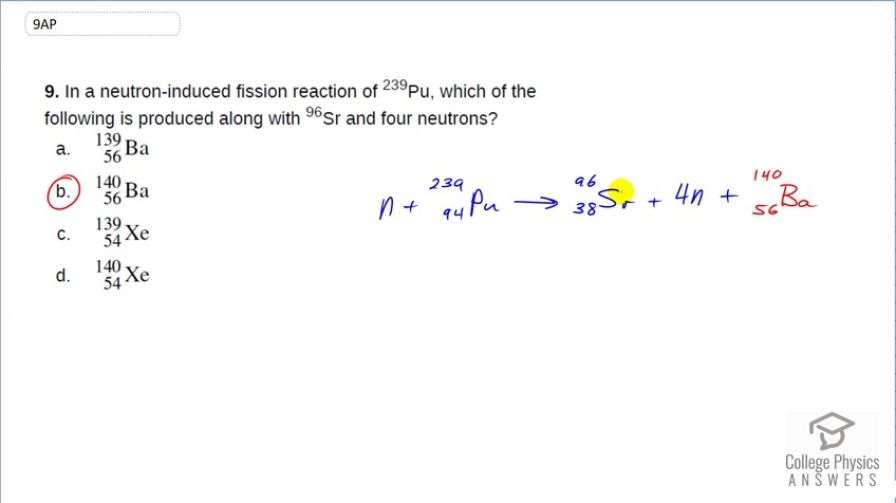
In a neutron-induced fission reaction of , which of the following is produced along with and four neutrons?
Final Answer
(b)
Solution video
OpenStax College Physics for AP® Courses, Chapter 32, Problem 9 (Test Prep for AP® Courses)
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. This is a neutron-induced fission reaction of plutonium-239 and we are told that it produces strontium-96 and four neutrons. So to figure out what else is produced, we have to use the law of conservation of nucleons to figure out what it is. So we know that we have 239 nucleons to start with plus one so that's 240 in total and then we have a total of 100 nucleons mentioned already between the strontium-96 and the four neutrons. So that means there are a 140 nucleons left over for whatever other product is created. And to figure out what that product is, we need to figure out its atomic number and we have 94 protons to start with; 38 of which are used for strontium leaving 56 left over for this thing and the material with an atomic number 56 is barium. So barium-140 is produced. The answer is (b).