Question
What is the energy obtained when 10 g of mass is converted to energy with an efficiency of 70%?
Final Answer
(a)
Solution video
OpenStax College Physics for AP® Courses, Chapter 32, Problem 8 (Test Prep for AP® Courses)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
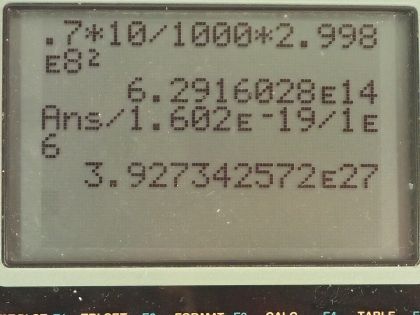
This is College Physics Answers with Shaun Dychko. Suppose 10 grams of mass is converted into energy with an efficiency of 70 percent, what is the energy obtained? Efficiency is the energy output divided by energy created or input and we are looking for the energy out so we multiply both sides by E in and we get then that the E out is the efficiency times the energy created— the energy created is the change in mass or this 10 grams multiplied by c squared. So we have 0.70—expressing 70 percent as a decimal, 0.70—times 10 grams converted into kilograms by dividing by a 1000 and then multiply by the speed of light squared so that gives 6.2916 times 10 to the 14 joules and then we convert our answer into megaelectron volts since our possibilities all have units of megaelectron volts. So we multiply by 1 electron volt for every 1.602 times 10 to the minus 19 joules and then multiply by 1 megaelectron volt for every 10 to the 6 electron volts and that is 3.93 times 10 to the 27 megaelectron volts and that is option (a).