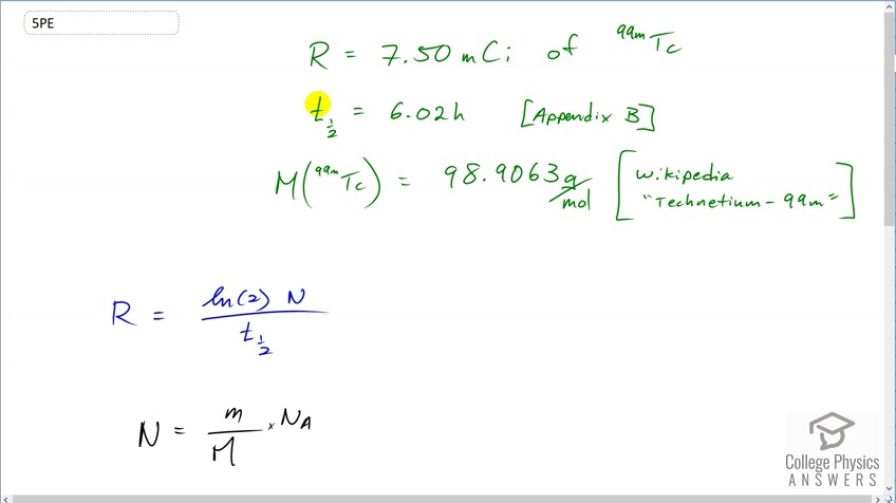
Question
Table 32.1 indicates that 7.50 mCi of is used in a brain scan. What is the mass of technetium?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 32, Problem 5 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. A brain scan uses technetium-99 with an activity of 7.50 millicuries and technetium-99 has a half-life of 6.02 hours according to appendix B of the textbook and a molar mass of 98.9063 grams per mol according to Wikipedia. Now what mass of technetium is being used is the question? Well activity is the natural logarithm of 2 multiplied by the number of nuclides divided by the half-life. And we can create an expression for the number of nuclides based on this here which says that the total mass divided by the molar mass multiplied by Avogadro's number is the number of nuclides. And you can verify that according to dimensional analysis or, in other words, looking at units we can verify that this is true and mass would be in grams and then we are gonna divide by grams per mol which is the same as multiplying by mols per gram; I like to keep everything on one line instead of dividing a fraction by a fraction so that's why I multiplied by mols per gram instead of dividing by grams per mol. And then multiplying by Avogadro's number which is number of atoms per mol and you see that the grams cancel and so do the mols leaving us with number of atoms. So this formula tells us the number of technetium atoms or nuclides. And we'll substitute that in place of N in our formula for activity. Then we'll solve for little m which is the mass and then we are multiplying both sides by the molar mass and the half-life and dividing both sides by natural logarithm of 2 and Avogadro's number and we end up with this line here. So the mass then is the activity times the molar mass times the half-life divided by natural logarithm of 2 times Avogadro's number. So that's 7.50 millicuries written as times 10 to the minus 3 curies and we have to convert that into becquerels since becquerels is an mks unit— meters, kilograms, seconds— or an SI unit you might also say— Système Internationale— and the curies cancel leaving us with becquerels and then we multiply that by 98.9063 grams per mol and then times that by the half-life expressed in seconds again because seconds are an mks unit. And divide by natural logarithm 2 and times by Avogadro's number and this gives 1.42 times 10 to the minus 9 grams. So 1.42 nanograms of technetium-99 is used to do this brain scan.
