Question
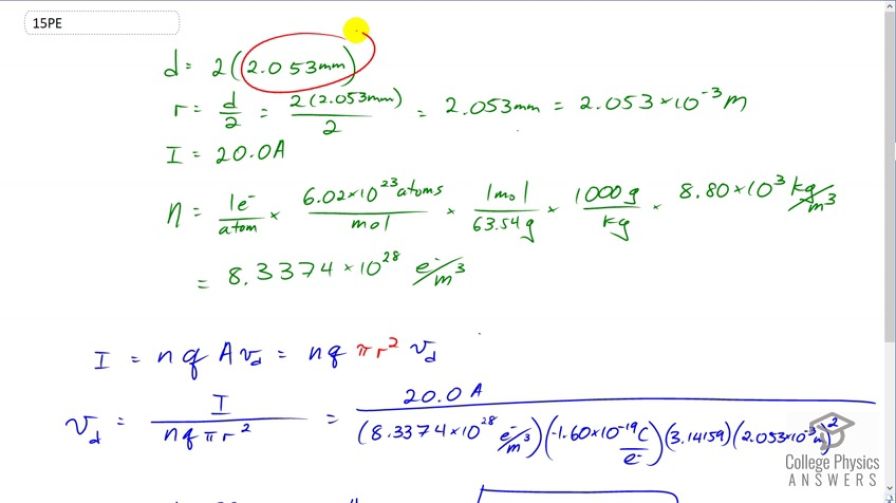
Using the results of Example 20.3, find the drift velocity in a copper wire of twice the diameter and carrying 20.0 A.
Example 20.3
Calculate the drift velocity of electrons in a 12-gauge copper wire (which has a diameter of 2.053 mm) carrying a 20.0-A current, given that there is one free electron per copper atom. (Household wiring often contains 12-gauge copper wire, and the maximum current allowed in such wire is usually 20 A.) The density of copper is .Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 20, Problem 15 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We're going to find the drift velocity of electrons in a copper wire that has a diameter that is twice what it was in example 20.3. So in example 20.3 the diameter is 2.053 millimeters, that's the diameter of twelve gauge household wire. So the radius will be half of that and I'm interested in the radius because the formula for the cross-sectional area of the wire is pi times r squared say. We could do pi d squared over four if you like but that's the way I like to write it. So the radius is going to be the diameter divided by two and we convert that into meters. So we have 2.053 times ten to the minus three meters. The current is 20 amps and the density of the charge carriers is figured out by all of this, so let's dig into that. We have one free electron per atom, this is something we're told about copper that it has one free electron in every atom. Then I multiply by 6.02 times ten to the twenty-three atoms for every mole and we're doing this because we want to get the volume density of the charge carriers. We're given the mass density of copper and we need to convert from these number of atoms into moles because we know a connection between the moles and the mass. If we look in the index at the back of the textbook it tell us this is one mole for every 63.54 grams. The units will all work out to number of electrons per cubic meter. So we can see that there's atoms on top and bottom there, moles on the top and bottom there, we converted the grams into kilograms because we have our mass density provided in kilograms per cubic meter so kilograms will cancel there. We're left with number of electrons per cubic meter. That's 8.3374 times ten to the twenty-eight electrons per cubic meters. There we go! Then we take this formula for the current in terms of drift velocity and we will solve it for drift velocity by dividing both sides by n Q pi r squared. Drift velocity is current divide by n Q pir squared. Q is the amount of charge on each charge carrier in this material. Since the charge carriers are electrons this is going to be just the elementary charge. So this is 20 amps divided by 8.3374 times ten to the twenty-eight density of charge carriers, multiplied by the number of coulombs per charge carrier, that's negative 1.6 times ten to the minus nineteen, times by pi times the radius, 2.053 times ten to the minus three meters squared. This gives negative 0.113 millimeters per second is the drift velocity. The negative means that the electrons are going in the opposite direction to the conventional current.
