Question
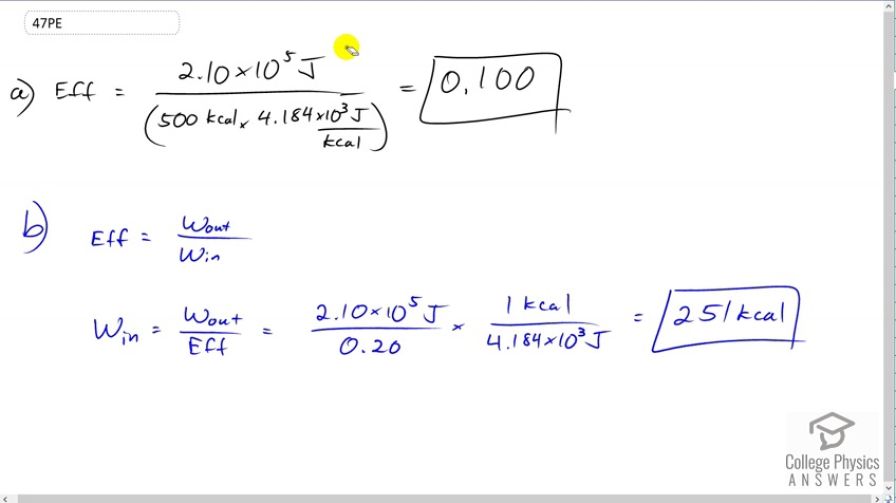
(a) What is the efficiency of an out-of-condition professor who does 2.10 \times 10^5 \textrm{ J} of useful work while metabolizing 500 kcal of food energy? (b) How many food calories would a well-conditioned athlete metabolize in doing the same work with an efficiency of 20%?
Final Answer
a)
b)
Solution video
OpenStax College Physics for AP® Courses, Chapter 7, Problem 47 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. Efficiency is the energy output divided by the energy input. So we have to convert the energy input of 500 kilocalories into joules, so multiply by 4.184 times ten to the three joules per kilocalorie and we get an efficiency of about ten percent, 0.100. In part B, we'll write down this formula that says efficiency is the energy output divided by the energy input. I've written the letter w for work because that's usually how the energy is manifested as in work, at least on the output side anyhow. So, we'll solve this for work in or energy input by multiplying by w in over efficiency on both sides. We get the work input is the work output divide by efficiency. So the output is 2.1 times ten to the five joules and we're told that the efficiency is now 20 percent or 0.2. Then we multiply by one kilocalorie for every 4.184 times ten to the three joules and we end up with 251 kilocalories.
