Solution video
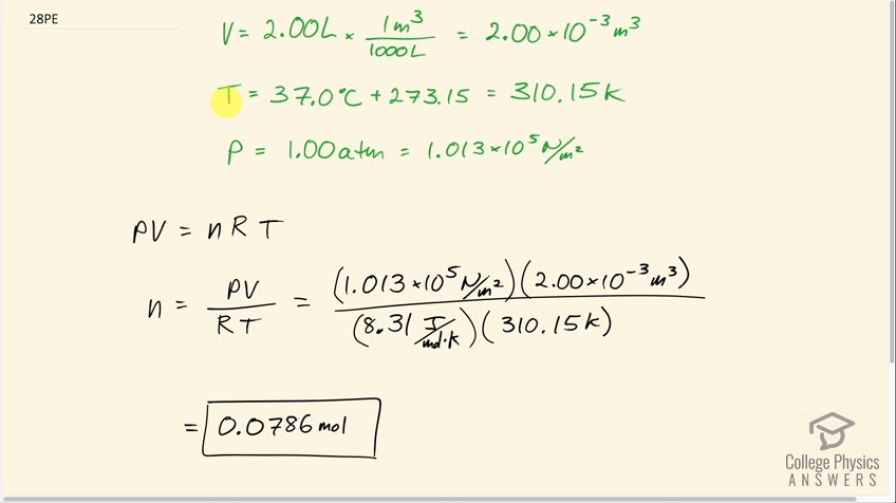
OpenStax College Physics, Chapter 13, Problem 28 (Problems & Exercises)
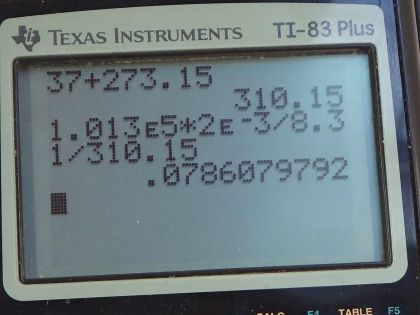
Calculator Screenshots
Comments
The answer you show is wrong from the video... .0786 in video but solution give is .786 mol big difference.
Hi Shaun! Thank you for answering my previous question, but I am still confused with when to use 1 ATM vs 1.013x105 N/m2 as in the previous question you said use 1 ATM but now are saying to use MKS units and shouldn't use 1ATM. How am I supposed to choose which unit to use?
Hi Christina,
Just for reference for other students, your original question was at https://collegephysicsanswers.com/openstax-solutions/suppose-gas-filled…. The question is how to know which units to use. "atm" vs "N/m^2". It's always correct to use "N/m^2", so if you want a single never-fail rule, then go with "N/m^2". However, it's easier to conceptualize a pressure expressed in units of "atm" since we can say "ah, so 1.5 atm is one and a half times the air pressure I experience at sea level". We have experience with "atm", so it's nice, although not any more correct, to use "atm" when possible. You'll see many of the solutions here using "atm". So the question becomes - when is it OK to use "atm" units? The answer is that one needs to look at the formula and see whether a factor in units of "atm" is being combined with any other number with different units. Often, as in problem 24, the pressure, in "atm", is being multiplied by a dimensionless factor (it has no units) in which case "atm" is fine. It was being multiplied by Kelvin divided by Kelvin, which therefore has no units since they cancel.
Hope this helps,
Shaun
Hi, am i missing something? In the equation you are multiplying R and T but when you entered it in the calculator you divided by T?
Hi kkm, thank you for the question. The issue is a personal preference about how to type this into the calculator. To save some button pushing I don't usually enclose the denominator in brackets. If there were brackets for the denominator, then you would see R and T multiplied. However, without brackets, and knowing the calculator proceeds from left to right, I divide by each factor in the denominator, one after the other. As an example to explain: 12 / (6 * 2) = 12 / 6 / 2 = 1. For 12 / 6 / 2, the calculator evaluates 12 / 6 first, which is "2", and then proceeds to divide that result by 2, giving the answer 1 We're on the same page, but just enter it differently in the calculator.
All the best,
Shaun
Hi,
When presented with this question. How do you know that 1 atm is the pressure of lungs. Is this something that you are supposed to just know? When I set up my formula, since the question doesn't say anything about pressure, how do you know to assume the pressure is 1 atm?
Hi,
When presented with this question. How do you know that 1 atm is the pressure of lungs. Is this something that you are supposed to just know? When I set up my formula, since the question doesn't say anything about pressure, how do you know to assume the pressure is 1 atm?