Question
The vapor pressure of water at is . Using the ideal gas law, calculate the density of water vapor in that creates a partial pressure equal to this vapor pressure. The result should be the same as the saturation vapor density at that temperature ().
Final Answer
Solution video
OpenStax College Physics, Chapter 13, Problem 58 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
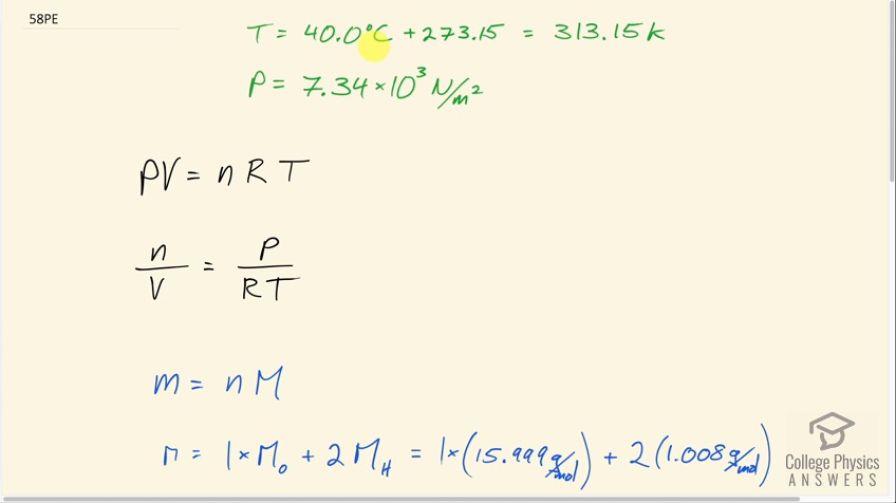
This is College Physics Answers with Shaun Dychko. Knowing the vapor pressure of water being 7.34 times ten to the three newtons per square meter and the temperature we're going to figure out what the density is of the water vapor in the air in grams per cubic meter. So this vapor pressure... this happens when there is an equilibrium between the amount of vapor and the water that's below it. And so, there's an equal amount of water turning into vapor as there is vapor turning into water. That's a dynamic equilibrium. And so, when that happens, you ha ve the vapor pressure and that's going to be the maximum amount of after that can go into the air at that temperature because anything more than that will cause more water to condense or any less than that will cause more water to evaporate. So at this special particular pressure called vapor pressure you have that equilibrium. And so that's the maximum density of water that corresponds to the maximum density of water vapor in the air. All right. We convert the temperature into Kelvin by adding 273.15 and then we're going to use P V equals N R T to figure out what the density is. So we'll solve this for the number of moles per volume and then we'll convert moles into a mass later. So we can divide both sides by V to get N divided by V. And then also divide both sides by the universal gas constant and temperature to get P divided by R T on one side and N over V on the other side. So then we have to relate this N number of moles to mass and mass is going to be the number of moles multiplied by the molar mass of the water. So this is the number of grams per mole. So grams per mole not multiplied by moles equals grams. So the molar mass will be the molar mass of the one oxygen atom in a water molecule because the formula is H2O and add to that 2 hydrogen. So we have one times 15.999 grams per mole for oxygen plus two times 1.008 grams per mole for hydrogen. And that's 18.015 grams from all in total for water. And so the number of moles we can solve for by dividing both sides by this molar mass M. It's going to be small M divided by capital M and this can be substituted in place of N here and we do that in red here. So we have mass divided by molar mass all over V equals P over R T. Now if we multiply this side by molar mass, this M will cancel with this M, and then we have to do the same to the other side until we have this line here. So mass divided by volume which is density - that's what mass over volume is - that's the vapor density in this case, equals the pressure times the molar mass of water divided by the universal gas constant times the absolute temperature. So it's 7.34 times ten to the three newtons per square meter times 18.015 grams per mole divided by 8.31 joules per mole Kelvin 313.15 Kelvin and that's 50.8 grams per cubic meter. And that's pretty close to what we're given in table 13.5 which says at 40 degrees Celsius we should have a saturation vapor density of 51.1 and 50.8 is pretty close to that.
