Question
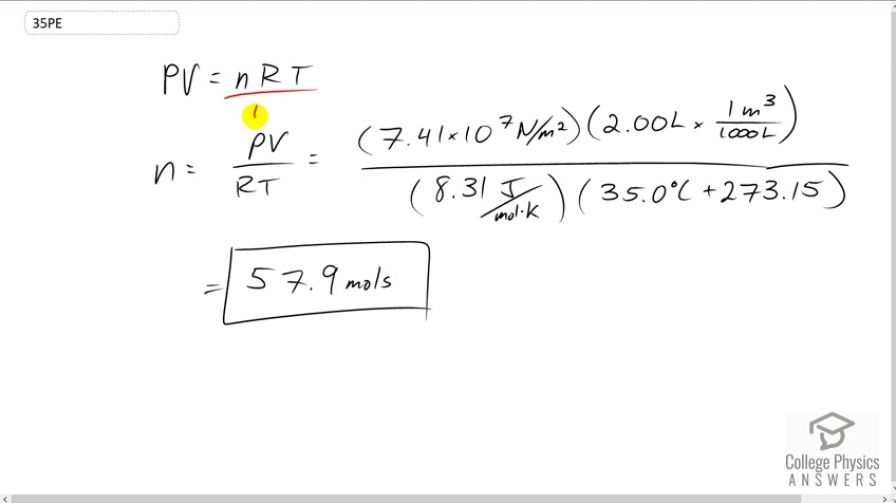
Find the number of moles in of gas at and under of pressure.
Final Answer
Solution video
OpenStax College Physics, Chapter 13, Problem 35 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. Pressure times volume equals the number of moles times the gas constant times the temperature in Kelvin. We can divide both sides by R T to solve for n. So n is P V over R T. The pressure is 7.41 times ten to the seven newtons per square meter, times two liters converted into cubic meters in order to be consistent with the meters squared that are there. So we multiply by one cubic meter for every thousand liters, and all this is divided by the gas constant which is 8.31 joules per mole Kelvin, times the temperature 35 degrees Celsius converted into Kelvin by adding 273.15. This makes 57.9 moles of gas.
