Question
Confirm that charge, electron family number, and the total number of nucleons are all conserved by the rule for α decay given in the equation . To do this, identify the values of each before and after the decay.
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics, Chapter 31, Problem 30 (Problems & Exercises)
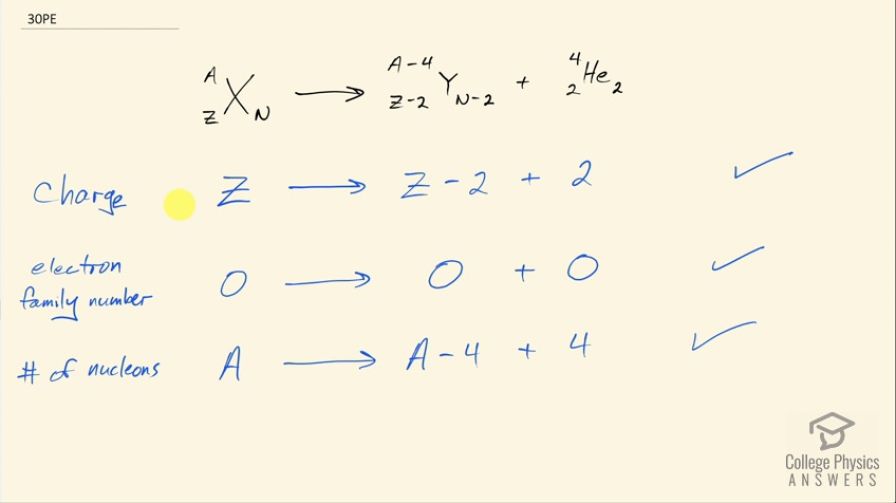
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. In α decay, we are going to verify that these three conservation rules are followed. So first is the conservation of charge that says the total charge before the reaction has to equal the total charge after and before, the nuclide has a positive Z charge and after the α decay, we have an α particle that has a charge of positive 2 and this daughter nuclide with a charge of 2 less than what this parent nuclide had so 2 less than Z and then Z minus 2 plus 2 is Z and so yes, charge is conserved. The electron family number is 0 on the left and it's zero for each of these particles on the right and so the electron family number is conserved; only electrons and electron-neutrinos and their anti-matter counterparts have electron family numbers. The total number of nucleons has to be conserved and initially in the parent nuclei, there are A nucleons— that's the sum of the protons plus neutrons— and on the right hand side, we have 4 less than the original A in this daughter nuclide but then we have 4 added in this α particle and so A minus 4 plus 4 is still A and so we have A on the left and a total of A on the right and so that means the number of nucleons is conserved.