Question
Write the complete decay equation for the given nuclide in the complete notation. Refer to the periodic table for values of Z:
Electron capture by
Electron capture by
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics, Chapter 31, Problem 21 (Problems & Exercises)
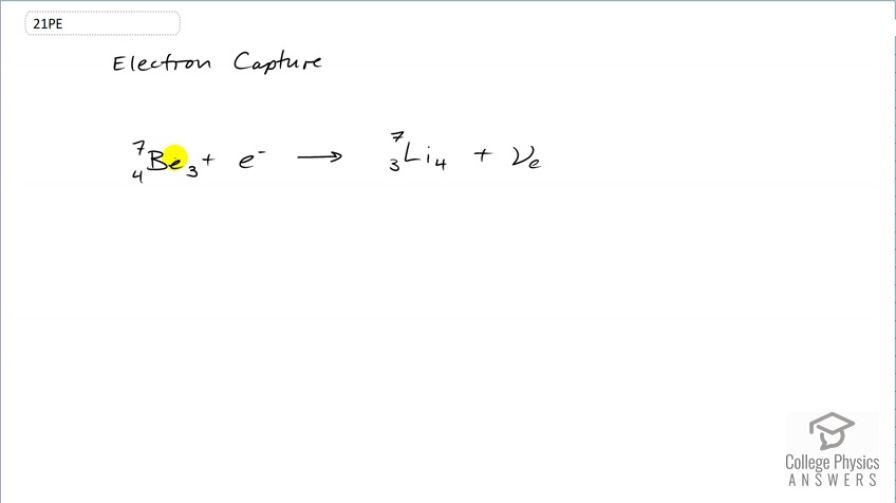
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. Electron capture happens when an electron gets absorbed by the nucleus and so this beryllium is going to have one of its protons neutralized by the absorption of this electron and so it turns into lithium which has only three protons in the nucleus. But the number of nucleons remains the same; they are still 7 in both cases but the lithium has one more neutron than the beryllium did because the electron plus one of the protons makes a neutron. Now since an electron has disappeared that means all this left side has an electron family number of 1 and on the right hand side, we need to also have an electron family number of 1 and if we didn't have this electron-neutrino here, there would be a violation of the conservation of electron family number because on the right side, there's nothing with a electron family number of 1. But this neutrino shows up in order to conserve that value. So charge is conserved because we started with 4 plus negative 1 which is a total of 3 and we ended with positive 3 so that's good. And we talked about the electron family number being conserved; it's 1 on both sides and the number of nucleons is also conserved and there we go. That's the electron capture of beryllium-3 or beryllium-7 I should say.