Question
Identify the parent nuclide and write the complete decay equation in the notation: decay producing . The parent nuclide is in the decay series produced by , the only naturally occurring isotope of thorium.
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics, Chapter 31, Problem 28 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Video Transcript
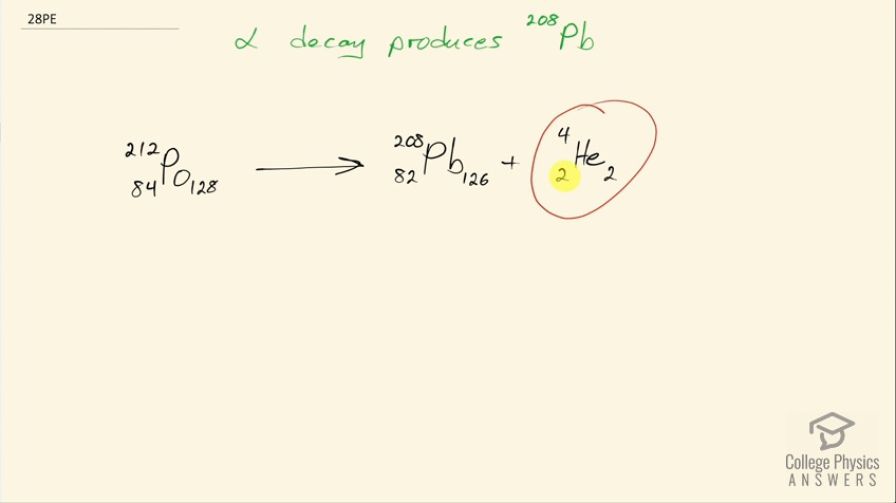
This is College Physics Answers with Shaun Dychko. α decay produces lead-208 so α decay produces an α particle, which is a helium nucleus— it has 2 protons and 2 neutrons for a total of 4 nucleons— and lead-208 has an atomic number of 82 which we can figure out from this appendix A here lead-208 has an atomic number of 82 so that means there are 82 protons, which leaves 126 neutrons left over when you subtract 82 from 208 total nucleons. So the α decay is the result of this parent nuclide shedding 2 protons and 2 neutrons in the form of this helium nucleus leaving behind this daughter nuclide that has 2 fewer protons 2 fewer neutrons than the parent nuclide. So that means there must have been 84 protons in the parent nuclide and 128 neutrons for a total of 212 nucleons and the element with an atomic number of 84 is polonium with a symbol 'Po'. There we go!