Question
Confirm that charge, electron family number, and the total
number of nucleons are all conserved by the rule for β− decay given in the equation . To do this, identify the values of each before and after the decay.
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics, Chapter 31, Problem 32 (Problems & Exercises)
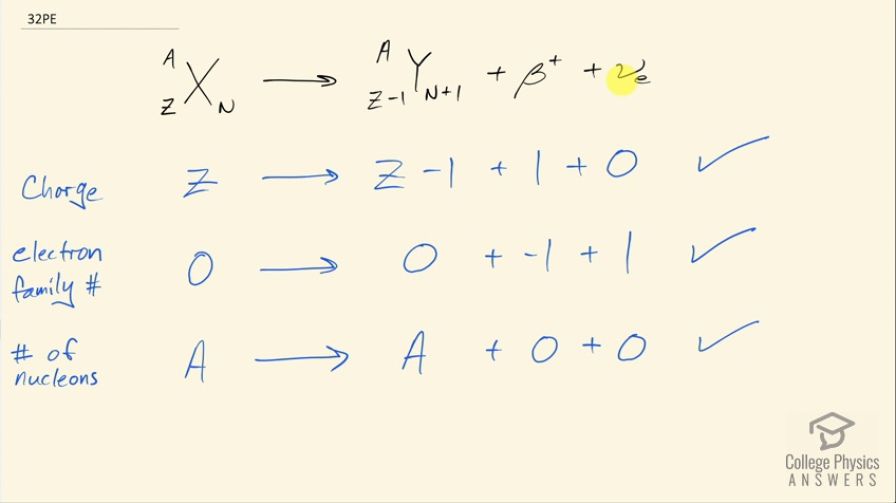
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. We are going to show that charge, electron family number and the total number of nucleons is conserved in positron decay so positron decay involves a proton turning into a positron, which is an anti-electron and then also into a neutron so we have 1 more neutron in this daughter nuclide and we have 1 fewer proton compared to the parent nuclide; there's also an electron-neutrino that is emitted as well So let's look at charge first: on the left side, we have a total of Z... is the number of protons so it's a charge of a positive Z and on the right hand side, we have 1 less than Z in the charge of this daughter nuclide but then we have that drop compensated for by a positive charge on this positron and there's zero charge in the electron-neutrino and so this checks out— charge is conserved because the total is the same before and after, it's Z in both cases. The electron family number is 0 for this parent nuclide and 0 for the dotted nuclide as well and it's negative 1 for the positron and it is positive 1 for the electron-neutrino and so the total is 0 on the right hand side just as it is on the left side so yes, it is conserved. And the total number of nucleons is A in the parent nuclide and it's also A in the daughter nuclide; the number of nucleons didn't change, it's just that one of the protons turned into a neutron but there's no change in the total number of nucleons.