Question
Write the complete decay equation for decay of in the complete notation:
Final Answer
Please see the solution video.
Solution video
OpenStax College Physics, Chapter 31, Problem 20 (Problems & Exercises)
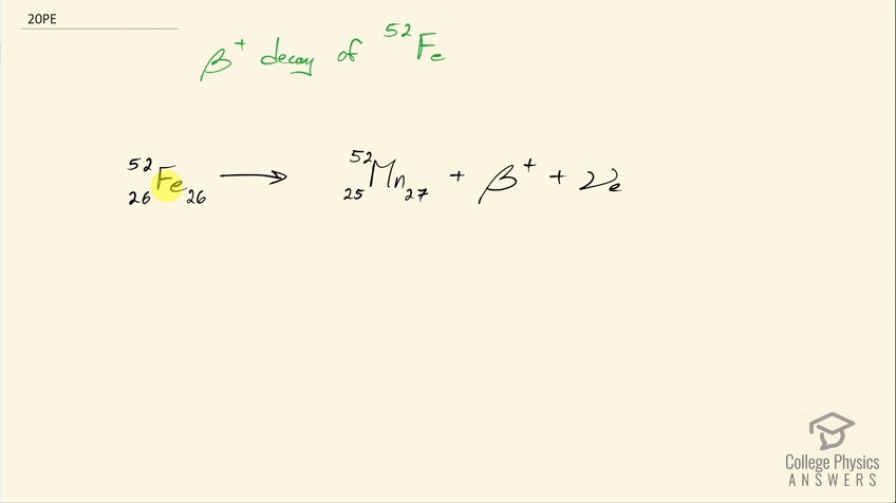
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. This formula shows positron decay of iron-52 we need to figure out how many protons are in iron by looking at the appendix at the end of the textbook and there are 26 protons because that's the atomic number of iron and there are a total of 52 nucleons and so that leaves 26 left over... must be neutrons. In positron decay, a proton turns into a neutron and a positron so there are now 25 protons so whereas there were 26 before, there are now 25 so we have taken away 1 and we have added 1 neutron and then that positron is emitted— and a positron is an example of anti-matter— so this electron-neutrino that's also emitted is a regular matter electron-neutrino so there's no bar on the top there, it's just regular matter.
