Question
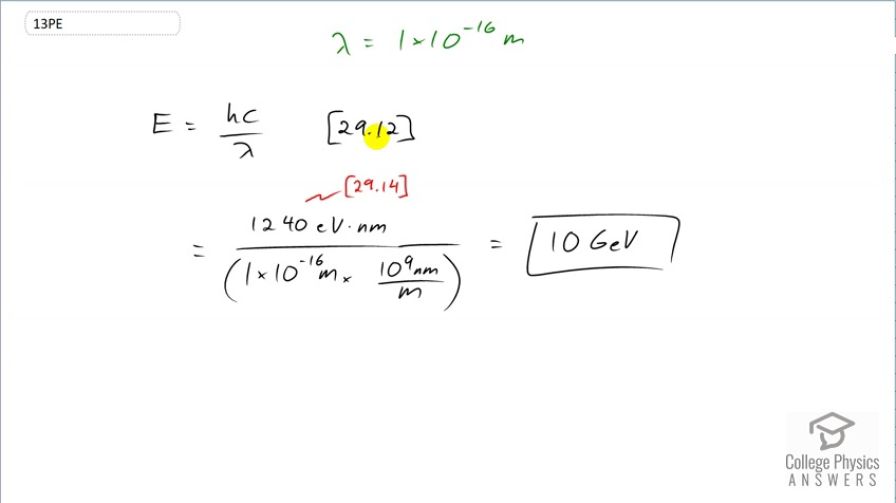
The detail observable using a probe is limited by its wavelength. Calculate the energy of a -ray photon that has
a wavelength of , small enough to detect details about one-tenth the size of a nucleon. Note that a photon having this energy is difficult to produce and interacts poorly with the nucleus, limiting the practicability of this probe.
Final Answer
Solution video
OpenStax College Physics, Chapter 31, Problem 13 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We want to know the energy of a photon that has a wavelength of 1 times 10 to the minus 16 meters. Equation [29.12] gives us the answer; it's gonna be Planck's constant times the speed of light divided by the wavelength and this product hc can be written as 1240 electron volt nanometers; that's what is told to us in chapter 29, equation 14. And when we divide this by the wavelength, we need to express the wavelength also in units of nanometers so that these nanometers cancel leaving us with units of electron volts in our answer. And so we'd divide by 1.1 times 10 to the minus 16 meters but then we have to convert that into nanometers by multiplying by 10 to the 9 nanometers per meter and we are left with nanometers in the bottom which then cancel with these nanometers up here. All of this works out to 10 gigaelectron volts will be the energy of this photon.
