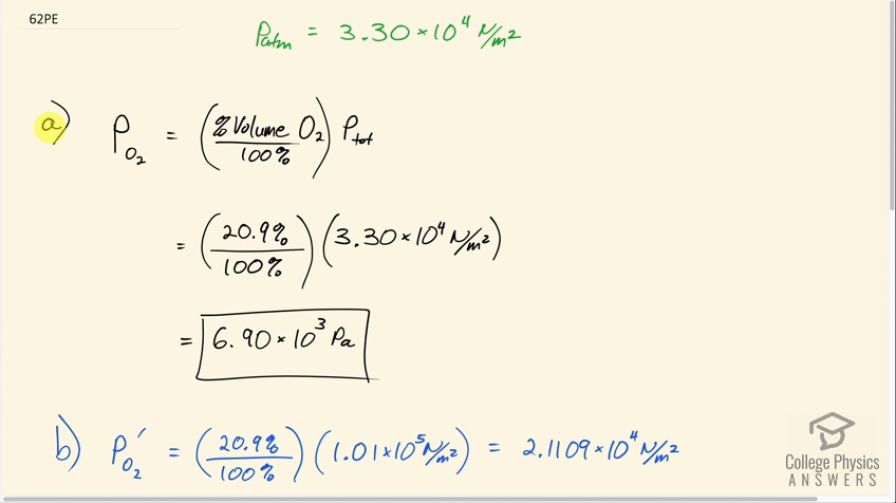
Question
Atmospheric pressure atop Mt. Everest is . (a) What is the partial pressure of oxygen there if it is 20.9% of the air? (b) What percent oxygen should a mountain climber breathe so that its partial pressure is the same as at sea level, where atmospheric pressure is
? (c) One of the most severe problems for those climbing very high mountains is the extreme drying of breathing passages. Why does this drying occur?
Final Answer
- The partial pressure of water vapor is lower at the low atmospheric pressures of high altitude. The more the partial pressure is below the vapor pressure, the greater the rate of evaporation. This greater rate of evaporation drys the breathing passages of the mountain climbers.
Solution video
OpenStax College Physics for AP® Courses, Chapter 13, Problem 62 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko The atmospheric pressure on top of Mount Everest is 3.3 times 10 to the 4 newtons per square meter. And question a) is asking us: what is the partial pressure of oxygen there? So we're given what the volume percent of oxygen is of the mixture of gases in the atmosphere and it's 20.9%. And so we take that percentage and convert it into a decimal, multiply it by the total pressure which is the atmospheric pressure and that gives us the partial pressure. So 20.9% divided by a hundred percent times 3.3 times 10 to the 4 newtons per square meter is 6.9 times 10 to the 3 Pascal's. So that's the partial pressureof oxygen top of Mount Everest where the atmospheric pressure is this. Now in Part B it's asking what percent oxygen should there be on the top of Mount Everest such that you get the same partial pressure of oxygen as you would get at sea level? So first we'll figure out what is that partial pressure of oxygen at sea level. And I've got this prime here to label this as partial pressure at sea level and the oxygen will make up the same percentage of the total air, so that's 20.9%. But now it's being multiplied by atmospheric pressure at sea level, so 1.01 times 10 to the 5 newtons per square meter for a partial pressure of 2.1109 times 10 to the 4 newtons per square meter. So the percent volume on top of Mount Everest is going to be... We divide this by P total and this by P total and then multiply by 100% and we end up with the percent volume of oxygen. And so that's what we have here. So we have 2.1109 times 10 to the 4 newtons per square meter is the partial pressure at sea level of oxygen divided by 3.3 times 10 to the 4 newtons per square meter total pressure on top of Mount Everest times 100% gives us 64.0%. So if the oxygen made up 64% of all the air on the top of Mount Everest then at that lower atmospheric pressure there you would still get the same partial pressure of 2.1 times ten to the four on the top of Mount Everest as you do at sea level. So this is why mountain climbers on top of Mount Everest carry oxygen bottles because they need to increase the percentage of the oxygen in air that they're breathing and if they increase it up to 64% then they're getting the same oxygen level as they would get at sea level. So Part C says that that extreme drying of breathing passages is a severe problem for these mountain climbers. So the partial pressure of water vapor decreases just the same as the partial pressure of everything else decreases as the total atmospheric pressure decreases with high altitude. And so with a really low partial pressure of water vapor they're going to get more dry because the more that the partial pressure is below the vapor pressure of water at the given temperature the more is the rate of evaporation.
