Question
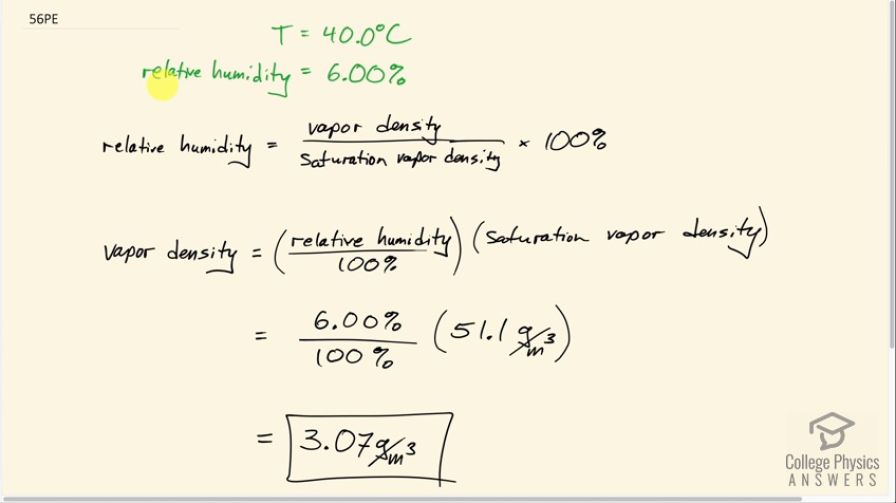
What is the density of water vapor in on a hot dry day in the desert when the temperature is and the relative humidity is 6.00%?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 13, Problem 56 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. We’re going to find the density of water vapor in the air when the temperature is 40 point zero degrees Celsius and the relative humidity is 6%. So relative humidity is the vapor density divided by the maximum possible vapor density at that temperature before it starts to rain if it goes beyond this saturation vapor density times 100%. So we'll solve for this by dividing both sides by 100 and then multiplying both sides by the saturation vapor density and then we get vapor density as relative humidity divided by 100 times saturation vapor density. So the saturation vapor density is something we need to look up in table 13.5. And so we follow the column of temperature down to 40 degrees Celsius and find that the saturation vapor density is 51.1 grams per cubic meter. So this is the maximum density of water vapor that's possible at this particular temperature and this works out to 3.07 grams per cubic meter.
