Question
Show that 60.0 L of gasoline originally at will expand to 61.1 L when it warms to as claimed in Example 13.4.
Final Answer
See solution video for verification.
Solution video
OpenStax College Physics for AP® Courses, Chapter 13, Problem 15 (Problems & Exercises)
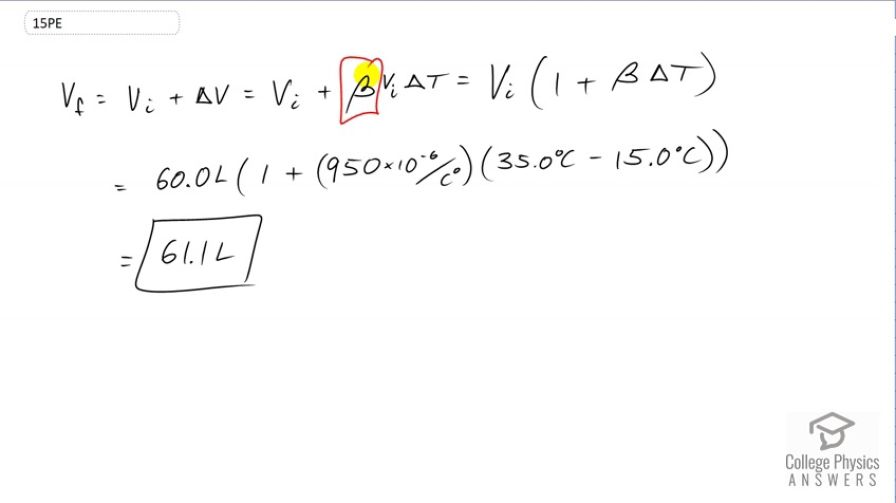
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. The final volume of this gasoline will be its initial volume plus its change in volume. The change in volume will be this coefficient of volume expansion times the initial volume, times the change in temperature. We can factor out V i from both these terms and we get V i equals one plus beta delta t. So that's 60 liters initial volume times one plus the volume expansion coefficient for petrol which is 950 times times ten to the minus six, then multiply by the change in temperature, 35 Celsius degrees minus 15. So that is 61.1 liters will be the final volume of the gasoline.
