Question
Suppose the relative humidity is 80% on a day when the temperature is . (a) What will the relative humidity be if the air cools to and the vapor density remains constant? (b) What is unreasonable about this result? (c) Which premise is responsible?
Final Answer
- 106%
- This is unreasonable since the maximum relative humidity is 100%
- As the temperature decreased from to rain would have occurred to reduce the vapor density and thereby keep the relative humidity less than or equal to 100%. The premise that vapor density is constant is false.
Solution video
OpenStax College Physics for AP® Courses, Chapter 13, Problem 72 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
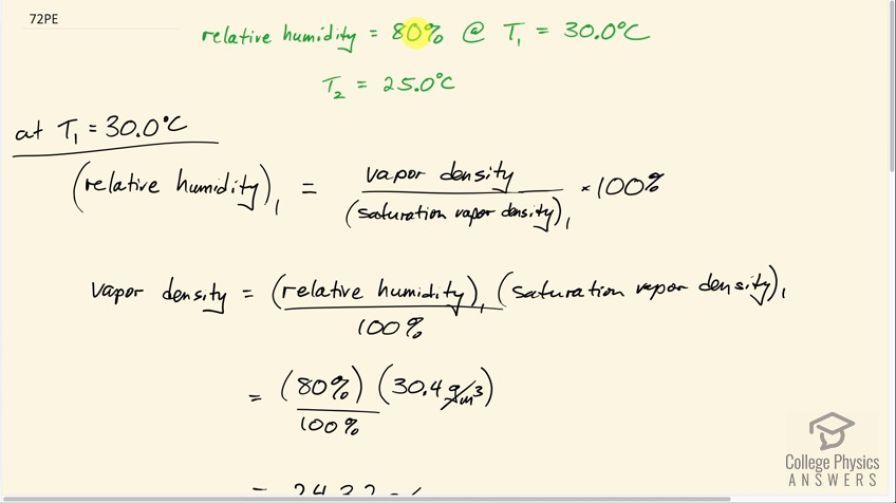
This is College Physics Answers with Shaun Dychko. The relative humidity at temperature one which is 30 degrees Celsius is 80%, and we're asked to find out what is the relative humidity going to be at temperature two, 25 degrees Celsius, assuming the same vapor density. So we have to figure out what the vapor density is. At temperature 1 we have relative humidity number 1 is the vapor density divided by the saturation vapor density times a hundred percent. And so we'll multiply both sides by the saturation vapor density at 30 degrees Celsius and then divide both sides by a hundred percent. And we get the vapor density then is the relative humidity multiplied by the saturation vapor density at 30 degrees divided by a hundred percent. So that's 80% divided by 100% times the saturation vapor density that we look up in table 13.5. So at 30 degrees Celsius, we have a saturation vapor density in grams per cubic meter of 30.4. So we plug that number in here and we get the vapor density is 24.32 grams per cubic meter. Now, at temperature 2 which is 25 degrees Celsius, the relative humidity then will be at that same vapor density we assume divided by the saturation vapor density at 25 degree Celsius times a hundred percent. So we have 24.32 grams per cubic meter divided by 23.0 grams per cubic meter since that's the saturation vapor density at 25 degrees Celsius times a hundred percent, which is 106 percent. So that's unreasonable because the maximum relative humidity is 100%. And so what's really going to happen is as the temperature decreases from 30 to 25 degrees Celsius rain would occur and that would reduce the vapor density and keep relative humidity at a maximum of a hundred percent. So the assumption that vapor density is constant is false.
