Question
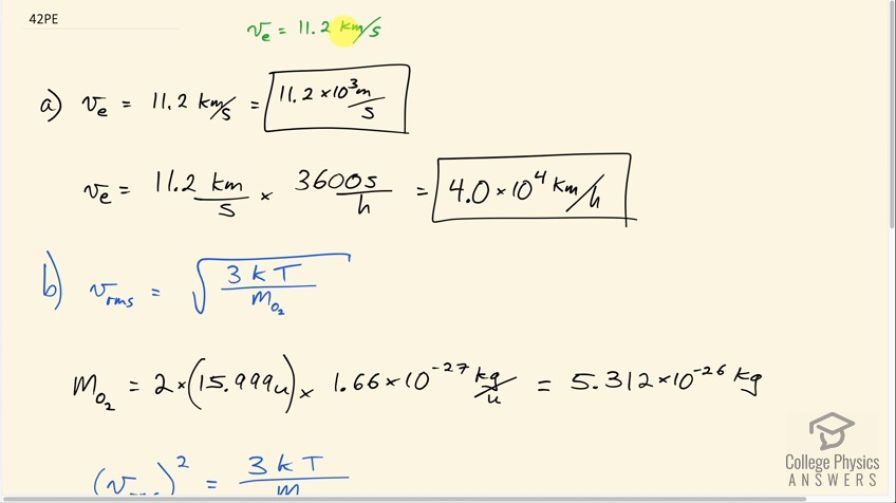
The escape velocity of any object from Earth is 11.2 km/s. (a) Express this speed in m/s and km/h. (b) At what temperature would oxygen molecules (molecular mass is equal to 32.0 g/mol) have an average velocity equal to Earth’s escape velocity of 11.1 km/s?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 13, Problem 42 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. The escape velocity from Earth is 11.2 kilometers per second. And part A asks us to express that speed in meters per second and kilometers per hour. So the prefix kilo means multiplied by 10 to the three. And so the conversion is just 11.2 times ten to the three meters per second. And to convert to kilometers per hour we take this 11.2 kilometers per second and multiply it by 3,600 seconds per hour giving us kilometers per hour. That's 4.0 times 10 to the four kilometers per hour. That's 40,000 kilometers an hour. Part B asks us: consider an oxygen molecule, what temperature would be needed such that the molecule would have this VRMS? So we need to know what the mass of an oxygen molecule is. So it's going to be two times the mass of an atom which if you look it up in a search engine you'll get 15. 999 atomic mass units which then have to be converted into kilograms by multiplying by 1.66 times ten to the minus 27 kilograms for every atomic mass unit giving 5.312 times ten to the minus 26 kilograms per oxygen molecule which consists of two atoms because oxygen is a diatomic molecule. So we square both sides of this formula because we were solving for T here and so we have V RMs squared equals 3 times Boltzmann's constant times temperature divided by mass of the molecule. And then we'll multiply both sides by M oh-2 over 3 K and then switch the sides around and we solve for T. So the temperature in Kelvin is mass of an oxygen molecule times V RMS squared over 3 times Boltzmann's constant. So that's 5.312 times ten to the minus 26 kilograms times 11.2 times ten to the three meters per second squared divided by three times 1.38 times ten to the minus 23 joules per Kelvin and that's a temperature of 1.61 times ten to the five Kelvin.

