Question
At a spot in the high Andes, water boils at , greatly reducing the cooking speed of potatoes, for example. What is atmospheric pressure at this location?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 13, Problem 54 (Problems & Exercises)
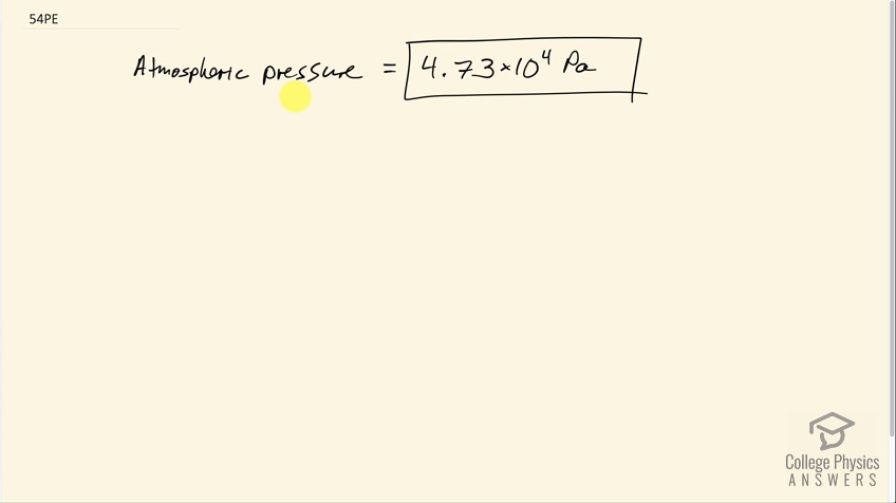
vote with a rating of
votes with an average rating of
.
Video Transcript
This is College Physics Answers with Shaun Dychko. On the Indian mountains water boils at 80 degrees Celsius, and the question is: what is the atmospheric pressure there? So we look in this table 13.5 and find 80 degrees Celsius in the temperature column and read what the vapor pressure is at that temperature. So that's 4.73 times 10 to the four. And if this vapor pressure is the same as the atmospheric pressure that's when boiling occurs. So, 100 degrees Celsius is normally considered the boiling point of water because at sea level where the atmospheric pressure is 1.013 and 10 to the five pascals that's when the vapor pressure of water becomes the same as the atmospheric pressure and the bubbles will come out of the water. But if the water is boiling at 80 degrees Celsius it means the atmospheric pressure there at the top of the mountain must be 4.73 times 10 to the four pascals.