Question
(a) In the deep space between galaxies, the density of atoms is as low as , and the temperature is a frigid 2.7 K. What is the pressure? (b) What volume (in ) is occupied by 1 mol of gas? (c) If this volume is a cube, what is the length of its sides in kilometers?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 13, Problem 38 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
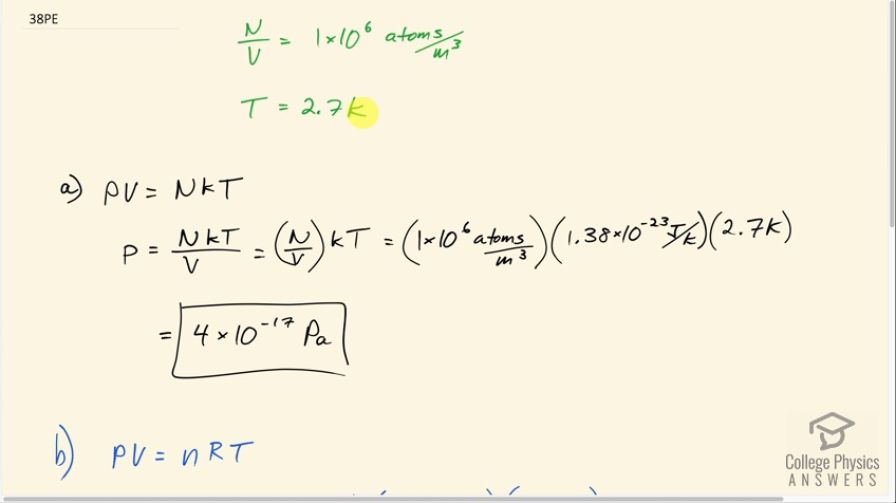
This is College Physics Answers with Shaun Dychko. In deep space there are about 1 times 10 to the 6 atoms per cubic meter at a temperature of 2.7 kelvin. And Part A this question asks: what will the pressure be in that case? So pressure times volume is the number of atoms times Boltzmann's constant times the absolute temperature. So we can find pressure by dividing both sides by volume and we have P equals n K T over V. Now we're given neither and nor V but we are given their ratio. So this is the same as this but I've written i>N over V in brackets here just to emphasize that this this ratio is this quantity that we're given. So we have 1 times 10 to the 6 atoms per cubic meter is this number density and multiply that by Boltzmann's constant times the temperature in kelvin and this gives 4 times 10 to the minus 17 Pascal's keeping only one significant since this number density is given with only one significant figure. In Part B we are asked what volume would one mole of gas take up? So we have this pressure that we've now calculated and we'll use that in this calculation for volume. So we have pressure times volume equals a small n which is number of moles times the universal gas constant R times T. And we're choosing this form of the ideal gas law because it has moles in it. So we'll divide both sides by P and we get V equals NRT over P. So we have the number of moles as 1 and we multiply it by i>R which is 8.31 joules per mole kelvin times 2.7 kelvin divided by the pressure written with lots of digits in order to avoid intermediate rounding error. So that's 3.726 times 10 to the minus 17 Pascal's. This gives the volume of six times 10 to the 17 cubic meters. And Part C, let's suppose that this volume is enclosed in a cube and the question is what would the side lengths of that cube be? So the volume of the cube is its side length to the power of 3. And to solve for s we raise both sides to the exponent one-third. And so the side length will be 6.022 times 10 to the 17 meters to the power of 1/3 and then we convert that into kilometers since the question asks for our answer in kilometers by multiplying by one kilometer for every thousand meters and we get 8 times 10 to the 2 kilometers which is about 800 kilometers.
