Question
Calculate the number of moles in the 2.00-L volume of air in the lungs of the average person. Note that the air is at (body temperature).
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 13, Problem 28 (Problems & Exercises)
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
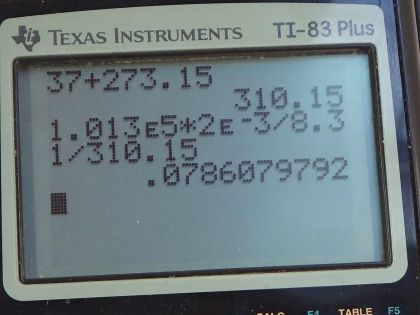
This is College Physics Answers with Shaun Dychko. We're going to calculate the number of moles of air molecules in the lungs of a person. So the temperature in the lungs is the core body temperature 37.0 degrees Celsius which we have to convert into kelvin in order to use it in our ideal gas law formula. So we add 273.15 to get a temperature of 310.15 kelvin. The volume we're told is two liters which we have to convert into cubic meters because we always want MKS units for formulas - meters, kilograms, and seconds. So we multiply by one cubic meter for every 1000 liters which is two times ten to the minus three cubic meters. The pressure we assume is about one atmosphere. If it was more than an atmosphere then they would be exhaling and if it was less than an atmosphere they'd be inhaling. So this is just the point between exhaling and inhaling which is the lungs are just filled up. And so the pressure is one atmosphere which we need to write in MKS units which is 1.013 times ten to the five newtons per square meter. So pressure times volume equals the number of moles times the universal gas constant times temperature and we can solve this for N by dividing both sides by R T So N is P V divided by R T. So that's 1.013 times ten to the five newtons per square meter pressure times two times ten to the minus three cubic meters ivided by 8.31 joules per mole kelvin and multiply that by 310.15 kelvin, and that's 0.0786 moles.