Question
(a) What is the average kinetic energy in joules of hydrogen atoms on the surface of the Sun? (b) What is the average kinetic energy of helium atoms in a region of the solar corona where the temperature is ?
Final Answer
Solution video
OpenStax College Physics for AP® Courses, Chapter 13, Problem 41 (Problems & Exercises)
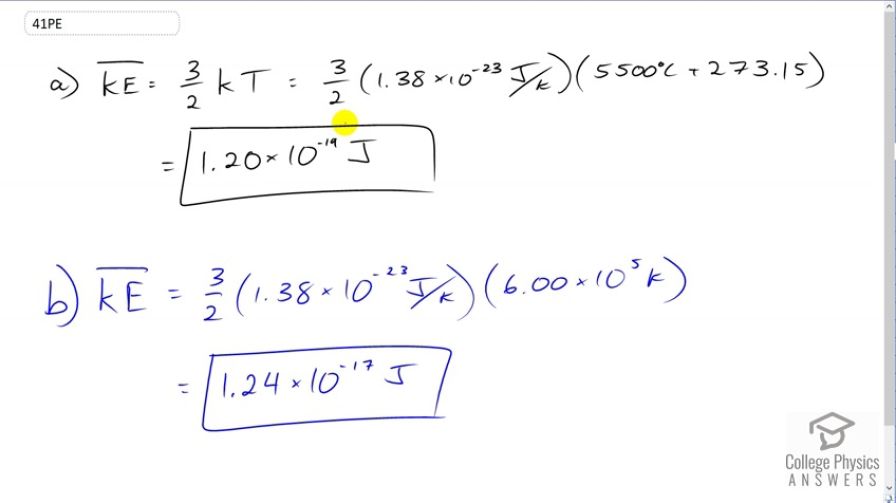
vote with a rating of
votes with an average rating of
.
Calculator Screenshots
Video Transcript
This is College Physics Answers with Shaun Dychko. Average kinetic energy is three over two times Boltzmann's constant times temperature in Kelvin. So that's three over two times 1.38 times ten to the minus twenty-three joules per Kelvin, times 5500 degrees Celsius, the surface of the sun converted into Kelvin by adding 273.15. This works out to 1.20 times ten to the minus nineteen joules. So that's the average kinetic energy of hydrogen atoms. It doesn't have to be hydrogen atoms; it could be any particle with that temperature. Now at this -- in the sun's corona where the temperature is six times ten to the five Kelvin, we multiply by Boltzmann's constant and times three over two and we get 1.24 times ten to the minus seventeen joules.
